CPT Codes for Transfusion Service Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Policy and Procedure
Policy and Procedure Title: Exchange Transfusion for Sickle Cell Division: Medical Management Disease Department: Utilization Management Approval Date: 2/9/18 LOB: Medicaid, Medicare, HIV SNP, CHP, MetroPlus Gold, Goldcare I&II, Market Plus, Essential, HARP Effective Date: 2/9/18 Policy Number: UM-MP224 Review Date: 1/18/19 Cross Reference Number: Retired Date: Page 1 of 7 1. POLICY: Exchange Transfusion for Sickle Cell Disease 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Claims Department, Provider Contracting 3. DEFINITIONS • Sickle cell disease – Sickle cell disease (SCD) refers to a group of inherited disorders characterized by sickled red blood cells (RBCs), caused either by homozygosity for the sickle hemoglobin mutation (HbSS; sickle cell anemia) or by compound heterozygosity for the sickle mutation and a second beta globin gene mutation (e.g., sickle-beta thalassemia, HbSC disease). In either HbSS or compound heterozygotes, the majority of Hgb is sickle Hgb (HgbS; i.e., >50 percent). • Transfusion – Simple transfusion refers to transfusion of RBCs without removal of the patient's blood. • Exchange Transfusion – Exchange transfusion involves transfusion of RBCs together with removal of the patient's blood. Exchange transfusion can be performed manually or via apheresis (also called cytapheresis or hemapheresis) using an extracorporeal continuous flow device. 4. PROCEDURE: A. Exchange transfusion for sickle cell disease will be covered as an ambulatory surgery procedure when all the following criteria are met: i) The member has documented SCD. ii) The exchange transfusion is a pre-scheduled procedure. iii) The purpose of the exchange transfusion is to prevent stroke, acute chest syndrome, or recurrent painful episodes. -

Sickle Cell Disease: Chronic Blood Transfusions
Sickle Cell Disease: Chronic Blood Transfusions There may be times when sickle cell patients require a blood transfusion. Such situations include preparing for surgery, during pregnancy, or during a severe complication such as an aplastic crisis, splenic sequestration or acute chest syndrome. In these cases, transfusion is a one-time intervention used to reduce the severity of the complication you are experiencing. However, if you have had a stroke, or an MRI or TCD shows that you are at high risk for having a stroke, your hematologist may recommend you begin chronic blood transfusions. What Does a Blood Transfusion Do? What are The Risks? Chronic (monthly) blood transfusions have been proven to Blood transfusions are not without risks. One risk is drastically reduce a sickle cell patient’s risk of stroke. They alloimmunization, a process in which the patient receiving have also been shown to reduce the frequency, severity blood transfusions creates antibodies to certain types of and duration of other sickle cell complications. Sickle cell blood. As a result he/she may have a reaction to the blood patients usually have a hemoglobin S level of about 80- that was transfused. Alloimmunization makes it more 90%. This means 80-90% of the circulating red blood cells difficult to find blood that is a good match for the patient. are cells that can sickle and cause complications. The goal In order to prevent alloimmunization, some centers of chronic blood transfusion therapy is to bring that routinely perform RBC phenotyping (special testing for percentage down below 30%. This will mean fewer sickle antibodies) on sickle cell disease patients so that they may cells circulating in the body, and a lower risk of give blood that is a better match for the patient. -
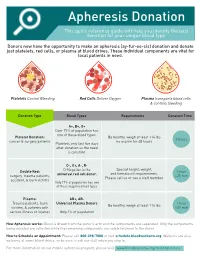
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Pediatric Orthotopic Heart Transplant Requiring Perioperative Exchange Transfusion: a Case Report
JECT. 2004;36:361–363 The Journal of The American Society of Extra-Corporeal Technology Case Reports Pediatric Orthotopic Heart Transplant Requiring Perioperative Exchange Transfusion: A Case Report Brian McNeer, BS; Brent Dickason, BS, RRT; Scott Niles, BA, CCP; Jay Ploessl, CCP The University of Iowa Hospitals and Clinics, Iowa City, Iowa Presented at the 41st International Conference of the American Society of Extra-Corporeal Technology, Las Vegas, Nevada, March 6–9, 2003 Abstract: An 11-month-old patient with idiopathic cardio- the venous line just proximal to the venous reservoir while si- myopathy was scheduled for orthotopic heart transplantation. A multaneously transfusing the normalized prime at normother- perioperative exchange transfusion was performed because of mia. Approximately 125% of the patients calculated blood vol- elevated panel reactive antibody levels. This process was accom- ume was exchanged. This technique greatly reduces the likeli- plished in the operating room prior to instituting cardiopulmo- hood of hyperacute rejection. The exchange transfusion process, nary bypass using a modified cardiopulmonary bypass circuit. In in addition to the patient immature immune system, provides preparation for the procedure, the cardiopulmonary bypass cir- additional options in orthotopic heart transplantation for pa- cuit was primed with washed leukocyte-filtered banked packed tients that may otherwise not be considered suitable candi- red blood cells, fresh-frozen plasma, albumin, and heparin. Pump dates. Keywords: exchange transfusion, heart transplant, pediat- prime laboratory values were normalized prior to beginning the ric, panel reactive antibodies. JECT. 2004;36:361–363 exchange transfusion. The patient’s blood was downloaded from Despite continuing advances in the management of end- humoral sensitization is determined by the presence of a stage cardiac failure, cardiac transplantation remains the positive panel reactive antibody (PRA) screen. -
Laboratory Best Transfusion Practice for Neonates, Infants and Children
Laboratory Best Transfusion Practice for Neonates, Infants and Children This summary guidance should be used in conjunction with the appropriate 20161 and 20122 BSH Guidelines and laboratory SOPs Compatibility testing Neonates and infants < 4 months Obtain neonatal and maternal transfusion history (including any fetal transfusions) for all admissions. Obtain a maternal sample for initial testing where possible, in addition to the patient sample. Red cell selection: no maternal antibodies present Select appropriate group and correct neonatal specification red cells. Group O D-negative red cells may be issued electronically without serological crossmatch. If the laboratory does not universally select group O D-negative red cells for this age group, blood group selection should either be controlled by the LIMS or an IAT crossmatch should be performed using maternal or neonatal plasma to serologically confirm ABO compatibility with both mother and neonate. Red cell selection: where there is maternal antibody Select appropriate group red cells, compatible with maternal alloantibody/ies. An IAT crossmatch should be performed using the maternal plasma. If it is not possible to obtain a maternal sample it is acceptable to crossmatch antigen-negative units against the infant’s plasma. Where paedipacks are being issued from one donor unit it is only necessary to crossmatch the first split pack. Subsequent split packs from this multi-satellite unit can be automatically issued without further crossmatch until the unit expires or the infant is older than 4 months. If packs from a different donor are required, an IAT crossmatch should be performed. Infants and children ≥ 4 months For infants and children from 4 months of age, pre-transfusion testing and compatibility procedures should be performed as recommended for adults. -

Cord Blood Stem Cell Transplantation
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights • Umbilical cord blood, like bone marrow and peripheral blood, is a rich source of stem cells for transplantation. There may be advantages for certain patients to have cord blood stem cell transplants instead of transplants with marrow or peripheral blood stem cells (PBSCs). • Stem cell transplants (peripheral blood, marrow or cord blood) may use the patient’s own stem cells (called “autologous transplants”) or use donor stem cells. Donor cells may come from either a related or unrelated matched donor (called an “allogeneic transplant”). Most transplant physicians would not want to use a baby’s own cord blood (“autologous transplant”) to treat his or her leukemia. This is because donor stem cells might better fight the leukemia than the child’s own stem cells. • Cord blood for transplantation is collected from the umbilical cord and placenta after a baby is delivered. Donated cord blood that meets requirements is frozen and stored at a cord blood bank for future use. • The American Academy of Pediatrics’s (AAP) policy statement (Pediatrics; 2007;119:165-170.) addresses public and private banking options available to parents. Among several recommendations, the report encourages parents to donate to public cord blood banks and discourages parents from using private cord blood banks for personal or family cord blood storage unless they have an older child with a condition that could benefit from transplantation. • The Stem Cell Therapeutic and Research Act of 2005 put several programs in place, including creation of the National Cord Blood Inventory (NCBI) for patients in need of transplantation. -

Therapeutic Apheresis, J Clin Apheresis 2007, 22, 104-105
Apheresis: Basic Principles, Practical Considerations and Clinical Applications Joseph Schwartz, MD Anand Padmanabhan, MD PhD Director, Transfusion Medicine Assoc Med Director/Asst Prof Columbia Univ. Medical Center BloodCenter of Wisconsin New York Presbyterian Hospital Medical College of Wisconsin Review Session, ASFA Annual meeting, Scottsdale, Arizona, June 2011 Objectives (Part 1) • Mechanism of Action • Definitions • Technology (ies) • Use • Practical Considerations • Math • Clinical applications – HPC Collection Objectives (Part 2) • Clinical applications: System/ Disease Specific Indications • ASFA Fact Sheet Apheresis •Derives from Greek, “to carry away” •A technique in which whole blood is taken and separated extracorporealy, separating the portion desired from the remaining blood. •This allows the desired portion (e.g., plasma) to be removed and the reminder returned. Apheresis- Mechanism of Action •Large-bore intravenous catheter connected to a spinning centrifuge bowl •Whole blood is drawn from donor/patient into the centrifuge bowl •The more dense elements, namely the RBC, settle to the bottom with less dense elements such as WBC and platelets overlying the RBC layer and finally, plasma at the very top. Apheresis: Principles of Separation Platelets (1040) Lymphocytes Torloni MD (1050-1061) Monocytes (1065 - 1069) Granulocyte (1087 - 1092) RBC Torloni MD Torloni MD Separate blood components is based on density with removal of the desired component Graphics owned by and courtesy of Gambro BCT Principals of Apheresis WBC Plasma Torlo RBC ni MD Torloni MD RBC WBC Plasma G Cobe Spectra Apheresis- Mechanism of Action Definitions • Plasmapheresis: plasma is separated, removed (i.e. less than 15% of total plasma volume) without the use of replacement solution • Plasma exchange (TPE): plasma is separated, removed and replaced with a replacement solution such as colloid (e.g. -

Apheresis Red Cell Exchange/Transfusions
APHERESIS RED CELL EXCHANGE/TRANSFUSIONS In a patient treated in Manchester, parasitemia was virtually eliminated over eight hours by a 3.5 liter exchange blood transfusion (Plasmodium Falciparum Hyperparasitemia: Use of Exchange Transfusion in Seven Patients and a Review of the Literature). Several cases of severe babesiosis refractory to appropriate antibiotic therapy have been reported to respond promptly and dramatically to red blood cell (RBC) exchange transfusion. Asplenic patients, however, generally have a more severe course of illness, with hemolytic anemia, acute renal failure, disseminated intravascular coagulation, and pulmonary edema. Primary therapy is with antibiotics including clindamycin and quinine, with RBC exchange transfusion reported to be effective in severe cases. The RBC exchange transfusions succeeded in reducing significantly the level of parasitemia, dramatically improving the condition of an extremely ill patient. Our report adds to the small but growing literature on severe Babesia infection in humans, and provides further evidence to support the use of RBC exchange transfusion to treat severe babesiosis. Its single great advantage over antibiotic therapy is its rapid therapeutic effectiveness (Treatment of Babesiosis by Red Blood Cell Exchange in an HIV-Positive Splenectomized Patient). There was rapid clinical improvement after the whole-blood exchange transfusion. In cases of severe babesiosis, prompt institution of whole-blood exchange transfusion, in combination with appropriate antimicrobial therapy, can be life-saving. In patients with progressive babesiosis, early intervention with exchange transfusion, along with appropriate antimicrobial therapy, should be considered to speed clinical recovery. (Fulminant babesiosis treated with clindamycin, quinine, and whole-blood exchange transfusion. However, asplenic patients may have a much more serious clinical course. -

Use of Blood Components in the Newborn
NNF Clinical Practice Guidelines Use of Blood Components in the Newborn Summary of recommendations • Transfusion in the newborn requires selection of appropriate donor, measures to minimize donor exposure and prevent graft versus host disease and transmission of Cytomegalovirus. • Component therapy rather than whole blood transfusion, is appropriate in most situations. • A clear cut policy of cut-offs for transfusions in different situations helps reduce unnecessary exposure to blood products. • Transfusion triggers should be based on underlying disease, age and general condition of the neonate. Writing Group : Chairperson: Arvind Saili ; Members: RG Holla, S Suresh Kumar Reviewers: Neelam Marwaha, Ruchi Nanawati Page | 129 Downloaded from www.nnfpublication.org NNF Clinical Practice Guidelines Introduction Blood forms an important part of the therapeutic armamentarium of the neonatologist. Very small premature neonates are amongst the most common of all patient groups to receive extensive transfusions. The risks of blood transfusion in today’s age of rigid blood banking laws, while infrequent, are not trivial. Therefore, as with any therapy used in the newborn, it is essential that one considers the risk- benefit ratio and strive to develop treatment strategies that will result in the best patient outcomes. In addition, the relatively immature immune status of the neonate predisposes them to Graft versus Host Disease (GVHD), in addition to other complications including transmission of infections, oxidant damage, allo- immunization and -

Transfusion Support Issues in Hematopoietic Stem Cell Transplantation Claudia S
Knowledge of transfusion complications related to HSCT can help with the early detection and treatment of patients before and after transplantation. Ray Paul. SP12-6796 × 40, 2013. Acrylic, latex, enamel on canvas printed with an image of myxofibrosarcoma with metastases to the artist’s lung, 26" × 36". Transfusion Support Issues in Hematopoietic Stem Cell Transplantation Claudia S. Cohn, MD, PhD Background: Patients receiving hematopoietic stem cell transplantation require extensive transfusion support until red blood cell and platelet engraftment occurs. Rare but predictable complications may arise when the transplanted stem cells are incompatible with the native ABO type of the patient. Immediate and delayed hemolysis is often seen. Methods: A literature review was performed and the results from peer-reviewed papers that contained reproducible findings were integrated. Results: A strong body of clinical evidence has developed around the common complications experienced with ABO-incompatible hematopoietic stem cell transplantation. These complications are discussed and the underlying pathophysiology is explained. General treatment options and guidelines are enumerated. Conclusions: ABO-incompatible hematopoietic stem cell transplantations are frequently performed. Immune-related hemolysis is a commonly encountered complication; therefore, health care professionals must recognize the signs of immune-mediated hemolysis and understand the various etiologies that may drive the process. Introduction antigen (HLA) matching remains an important predic- Hematopoietic stem cell transplantation (HSCT) is used tor of success with HSCT; however, the ABO barrier is to treat a variety of hematological and congenital diseas- often crossed when searching for the most appropriate es. The duration and specificity of transfusion support HLA match between donor and patient. -

Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods
Guidance for Industry Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods Additional copies of this guidance document are available from: Office of Communication, Training and Manufacturers Assistance (HFM-40) 1401 Rockville Pike, Rockville, MD 20852-1448 (Tel) 1-800-835-4709 or 301-827-1800 (Internet) http://www.fda.gov/cber/guidelines.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research (CBER) January 2001 Technical Correction February 2001 TABLE OF CONTENTS Note: Page numbering may vary for documents distributed electronically. I. INTRODUCTION ............................................................................................................. 1 II. BACKGROUND................................................................................................................ 1 III. CHANGES FROM THE DRAFT GUIDANCE .............................................................. 2 IV. RECOMMENDED DONOR SELECTION CRITERIA FOR THE AUTOMATED RED BLOOD CELL COLLECTION PROTOCOLS ..................................................... 3 V. RECOMMENDED RED BLOOD CELL PRODUCT QUALITY CONTROL............ 5 VI. REGISTRATION AND LICENSING PROCEDURES FOR THE MANUFACTURE OF RED BLOOD CELLS COLLECTED BY AUTOMATED METHODS.................. 7 VII. ADDITIONAL REQUIREMENTS.................................................................................. 9 i GUIDANCE FOR INDUSTRY Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods This