Donor Apheresis Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Requirements for Blood and Blood Components Intended for Transfusion Or for Further Manufacturing Use
Requirements for Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use Office of Blood Research and Review CBER, FDA Ask the FDA Session – AABB Annual Meeting October 26, 2015 1 Outline • Overview – Historical Background – Intent of the Rule – Organization of the Rule • Selected Provisions – Relevant Transfusion-Transmitted Infection – Control of Bacterial Contamination of Platelets – Medical Supervision – Donor Acknowledgement – Alternative Procedures – Donor Eligibility • Implementation 2 GAO Oversight and FDA Concerns • Publish in the form of regulations the guidelines that FDA deems essential to ensure the safety of the blood supply • Concern about the delay in requiring testing for emerging infectious agents, e.g. HTLV • Concern about blood safety and the regulations being out-of-date • Concerns about donor safety 3 Intent of the Final Rule • To better assure the safety of the blood supply and to help protect donor health • To make donor eligibility and testing requirements more consistent with current practices and to provide flexibility with regard to emerging infectious diseases • To accommodate technological advances • To establish requirements for donor education, donor history, and donor testing 4 Organization of the Final Rule - 1 • A. General • B. Definitions (§§ 606.3, 610.39, 630.3, 640.125) • C. Standard Operating Procedures (§ 606.100) • D. Control of Bacterial Contamination of Platelets (§ 606.145) • E. Records (§ 606.160) • F. Test Requirements (§§ 610.40, 640.5, 640.71(a)) • G. Donor Deferral (§ 610.41) 5 Organization of the Final Rule - 2 • H. Purpose and Scope (§ 630.1) • I. Medical Supervision (§ 630.5) • J. General Donor Eligibility Requirements(§ 630.10) • K. Donor Eligibility Requirements Specific to Whole Blood, Red Blood Cells and Plasma Collected by Apheresis (§ 630.15) • L. -

27. Clinical Indications for Cryoprecipitate And
27. CLINICAL INDICATIONS FOR CRYOPRECIPITATE AND FIBRINOGEN CONCENTRATE Cryoprecipitate is indicated in the treatment of fibrinogen deficiency or dysfibrinogenaemia.1 Fibrinogen concentrate is licenced for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenaemia and hypofibrinogenaemia,2 and is currently funded under the National Blood Agreement. Key messages y Fibrinogen is an essential component of the coagulation system, due to its role in initial platelet aggregation and formation of a stable fibrin clot.3 y The decision to transfuse cryoprecipitate or fibrinogen concentrate to an individual patient should take into account the relative risks and benefits.3 y The routine use of cryoprecipitate or fibrinogen concentrate is not advised in medical or critically ill patients.2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In the setting of major obstetric haemorrhage, early administration of cryoprecipitate or fibrinogen concentrate may be necessary.3 Clinical implications y The routine use of cryoprecipitate or fibrinogen concentrate in medical or critically ill patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of disseminated intravascular coagulopathy (MED-PP18, CC-PP7).2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In patients with critical bleeding requiring massive transfusion, suggested doses of blood components is 3-4g (CBMT-PP10)3 in adults or as per the local Massive Transfusion Protocol. -

Blood, Lymph, and Immunity Joann Colville
Blood, Lymph, and Immunity Joann Colville CHAPTER OUTLINE .»: BLOOD Function Introduction Lymphatic Structures Plasma THE IMMUNE SYSTEM Cellular Components of Blood Function Red Blood Cells Immune Reactions Platelets Immunization: Protection Against Disease White Blood Cells THE LYMPHATICSYSTEM Lymph Formation Characteristics LEARNING OBJECTIVES • List and describe the functions of blood • List the functions of the lymphatic system • Describe the composition of blood plasma • Describe the structure and functions of the lymph nodes, • Describe the characteristics of mature erythrocytes spleen, thymus, tonsils, and GALT Describe the structure of the hemoglobin molecule and • List the functions of the immune system explain the fate of hemoglobin following intravascular and • Differentiate between specific and nonspecific immune extravascular hemolysis reactions • Give the origin of thrombocytes and describe their • Differentiate between cell-mediated and humoral immunity characteristics and functions • List the components involved in cell-mediated immunity • Listthe types of leukocytes and describe the functions of each and explain the role of each • Describe the formation of lymph fluid and its circulation List and describe the classes of immunoglobulins through the lymphatic system • Differentiate between active and passive immunity BLOOD vessels of the cardiovascular system. It has three main func- tions: transportation, regulation, and defense. INTRODUCTION Blood. Say the word, and some people cringe, others faint, Function and if you believe authors Bram Stoker, Stephen King, and 1. Blood is a transport system. Christopher Moore (all authors of vampire books), others • It carries oxygen, nutrients, and other essential com- drool. But no matter what you think about blood, animals pounds to every living cell in the body. -

Transfusion Service Introduction Blood/Blood Products Requests and Turnaround Time Expectations
Transfusion Service Introduction All blood products and blood components are supplied to UnityPoint Health-Meriter Hospital by the American Red Cross Blood Services. Pathology consultation is available regarding blood and/or components and dosages. ABO and Rho(D)—specific type is used whenever possible for leuko-poor packed cell transfusions. ABO-compatible blood is used for all plasma and platelet components whenever possible. For any orders involving HLA-matched components, the patient must have been HLA typed (sent to the American Red Cross) a minimum of 48 hours prior to intended infusion of the component. HLA typing is only required once. Blood components that are thawed, pooled, washed, volume-reduced, or deglycerolized for a patient will be charged to the patient even if not transfused. The charge is done because these components may not be suitable for another patient. Examples include: Autologous or directed donations Pooled cryoprecipitate 4-hour expiration Thawed fresh frozen plasma 24-hour expiration Thawed cryoprecipitate 6-hour expiration Deglycerolized red cells 24-hour expiration Washed red cells 24-hour expiration All blood components must be completely infused within 4 hours of release from UnityPoint Health-Meriter Laboratories Blood Bank, or be infused within the expiration time. Refer to UnityPoint Health -Meriter’s Blood and Blood Products Transfusion Policy #123 for additional information located on MyMeriter. Blood/Blood Products Requests and Turnaround Time Expectations Requests from UnityPoint Health-Meriter Hospital are entered in the hospital computer system and print in the UnityPoint Health- Meriter Laboratories Blood Bank. For the comfort of the patient, it is important to coordinate collection for other tests with Blood Bank specimens. -

The Diagnosis of Paroxysmal Nocturnal Hemoglobinuria: Role of Flow Cytometry
THE DIAGNOSIS OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: ROLE OF FLOW CYTOMETRY Alberto Orfao Department of Medicine, Cancer Research Centre (IBMCC-CSIC/USAL), University of Salamanca, Salamanca, Spain Paroxysmal nocturnal hemoglobinuria (PNH) on one or multiple populations of peripheral blood is an acquired clonal disorder typically affecting red cells and/or leucocytes of PNH patients, this young adults, which involves the phosphatidylino- translating into a new diagnostic test. Because sitol glycan anchor biosynthesis class A gene of this flow cytometry became an essential tool (PIGA) coded in chromosome X, in virtually every in the diagnosis of PNH. In turn, it could be also case. The altered gene encodes for a defective pro- demonstrated that investigation of the presence of tein product, the pig-a enzyme which is involved GPI-deficient cells among circulating mature neu- in the early steps of the synthesis of glycosylphos- trophils and monocytes, increases the sensitivity phatidylinositol (GPI). This later molecule (GPI) of red blood cell screening because of the shorter acts as an anchor of a wide range of proteins to lifetime of both populations of leucocytes. Despite the cell surface. At present a large number of GPI- all the above, routine assessment of CD55 and anchor associated proteins have been described CD59 is also associated with several limitations which are expressed by one or multiple distinct due to distinct patterns of expression among dif- compartments of hematopoietic cells. The altered ferent cell populations, dim and heterogeneous PIGA gene leads to an altered GPI anchor which expression among normal individuals and the lack leads to defective expression of GPI-AP on the of experience in many labs as regards the normal cytoplasmic membrane, on the cell surface. -

Unintentional Platelet Removal by Plasmapheresis
Journal of Clinical Apheresis 16:55–60 (2001) Unintentional Platelet Removal by Plasmapheresis Jedidiah J. Perdue,1 Linda K. Chandler,2 Sara K. Vesely,1 Deanna S. Duvall,2 Ronald O. Gilcher,2 James W. Smith,2 and James N. George1* 1Hematology-Oncology Section, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma 2Oklahoma Blood Institute, Oklahoma City, Oklahoma Therapeutic plasmapheresis may remove platelets as well as plasma. Unintentional platelet loss, if not recognized, may lead to inappropriate patient assessment and treatment. A patient with thrombotic thrombocytopenic purpura- hemolytic uremic syndrome (TTP-HUS) is reported in whom persistent thrombocytopenia was interpreted as continuing active disease; thrombocytopenia resolved only after plasma exchange treatments were stopped. This observation prompted a systematic study of platelet loss with plasmapheresis. Data are reported on platelet loss during 432 apheresis procedures in 71 patients with six disease categories using three different instruments. Com- paring the first procedure recorded for each patient, there was a significant difference among instrument types ,than with the COBE Spectra (1.6% (21 ס P<0.001); platelet loss was greater with the Fresenius AS 104 (17.5%, N) .With all procedures, platelet loss ranged from 0 to 71% .(24 ס or the Haemonetics LN9000 (2.6%, N (26 ס N Among disease categories, platelet loss was greater in patients with dysproteinemias who were treated for hyper- viscosity symptoms. Absolute platelet loss with the first recorded apheresis procedure, in the 34 patients who had a normal platelet count before the procedure, was also greater with the AS 104 (2.23 × 1011 platelets) than with the Spectra (0.29 × 1011 platelets) or the LN9000 (0.37 × 1011 platelets). -

Platelet-Rich Plasmapheresis: a Meta-Analysis of Clinical Outcomes and Costs
THE jOURNAL OF EXTRA-CORPOREAL TECHNOLOGY Original Article Platelet-Rich Plasmapheresis: A Meta-Analysis of Clinical Outcomes and Costs Chris Brown Mahoney , PhD Industrial Relations Center, Carlson School of Management, University of Minnesota, Minneapolis, MN Keywords: platelet-rich plasmapheresis, sequestration, cardiopulmonary bypass, outcomes, economics, meta-analysis Presented at the American Society of Extra-Corporeal Technology 35th International Conference, April 3-6, 1997, Phoenix, Arizona ABSTRACT Platelet-rich plasmapheresis (PRP) just prior to cardiopulmonary bypass (CPB) surgery is used to improve post CPB hemostasis and to minimize the risks associated with exposure to allogeneic blood and its components. Meta-analysis examines evidence ofPRP's impact on clinical outcomes by integrating the results across published research studies. Data on clinical outcomes was collected from 20 pub lished studies. These outcomes, DRG payment rates, and current national average costs were used to examine the impact of PRP on costs. This study provides evidence that the use of PRP results in improved clinical outcomes when compared to the identical control groups not receiving PRP. These improved clinical out comes result in subsequent lower costs per patient in the PRP groups. All clinical outcomes analyzed were improved: blood product usage, length of stay, intensive care stay, time to extu bation, incidence of cardiovascular accident, and incidence of reoperation. The most striking differences occur in use of all blood products, particularly packed red blood cells. This study provides an example of how initial expenditure on technology used during CPB results in overall cost savings. Estimated cost savings range from $2,505.00 to $4,209.00. -
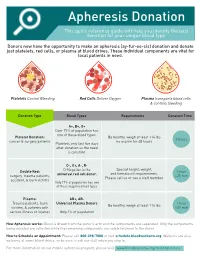
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Patient's Guide to Blood Transfusions
Health Information For Patients and the Community A Patient’s Guide to Blood Transfusions Your doctor may order a blood transfusion as part of your therapy. This brochure will focus on frequently asked questions about blood products, transfusions, and the risks and benefits of the blood transfusion. PLEASE NOTE: This information is not intended to replace the medical advice of your doctor or health care provider and is intended for educational purposes only. Individual circumstances will affect your individual risks and benefits. Please discuss any questions or concerns with your doctor. What is a blood transfusion? A blood transfusion is donated blood given to patients with abnormal blood levels. The patient may have abnormal blood levels due to blood loss from trauma or surgery, or as a result of certain medical problems. The transfusion is done with one or more of the following parts of blood: red blood cells, platelets, plasma, or cryoprecipitate. What are the potential benefits of a blood transfusion? If your body does not have enough of one of the components of blood, you may develop serious life-threatening complications. • Red blood cells carry oxygen through your body to your heart and brain. Adequate oxygen is very important to maintain life. • Platelets and cryoprecipitate help to prevent or control bleeding. • Plasma replaces blood volume and also may help to prevent or control bleeding. How safe are blood transfusions? Blood donors are asked many questions about their health, behavior, and travel history in order to ensure that the blood supply is as safe as it can be. Only people who pass the survey are allowed to donate. -

Changes to the Technical Manual, 18Th Edition Monday, November 17, 2014 12:00 P.M
Changes to the Technical Manual, 18th Edition Monday, November 17, 2014 12:00 p.m. – 1:30 p.m. (ET) / 5:00p.m. – 6:30 p.m. (GMT) When this file is opened, Acrobat Reader will, by default, display the slides including the Acrobat reader controls. To return to full screen mode, hit Ctrl-L on your computer keyboard or use your mouse to click View>Full Screen on the menu bar of the Acrobat Reader program. To take the slides out of full screen mode and display the Acrobat Reader controls, simply hit the Esc key on your computer keyboard. To advance slides during the program, use the Enter, Page Down, down arrow or right arrow on your computer keyboard. To back up slides during the program, use the Page Up, up arrow of left arrow on your computer keyboard. Please remember to logon to the Live Learning Center using your email address and password to complete an evaluation of the program and speakers. At this time, advance to the next slide and wait for the audioconference to begin. A 2014 Audioconference presented to you by AABB The AABB Technical Manual 18th Edition What’s new? www.aabb.org Technical Manual by the Numbers • 1953 =year of first edition • 69 = number of authors/editors this edition • 370,378 = word count • 96 = number of methods • 60 = number of countries where TM is used www.aabb.org It’s a Process www.aabb.org Overview of Changes Major • Molecular testing • Patient blood management • Cellular therapy • Methods Minor • Throughout www.aabb.org 1: Quality Systems 2: Facilities and Safety • These 2 chapters are comprehensive discussions -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Patient Information Leaflet – Plasma Exchange Procedure
Therapeutic Apheresis Services Patient Information Leaflet – Plasma Exchange Procedure Introduction Antibodies, which normally help to protect you from infection, can begin to attack your own This leaflet has been written to give patients healthy cells, or an over production of proteins can information about plasma exchange (sometimes cause your blood to become thicker and slow down called plasmapheresis). If you would like any more the blood flow throughout your body. A plasma information or have any questions, please ask the exchange can help improve your symptoms, doctors and nurses involved in your treatment at although this may not happen immediately. the NHS Blood and Transplant (NHSBT) Therapeutic Apheresis Services Unit. Although plasma exchange may help with symptoms, it will not normally cure your condition When you have considered the information given as it does not switch off the production of the in this leaflet, and after we have discussed the harmful antibodies or proteins. It is likely that procedure and its possible risks with you, we will this procedure will form only one part of your ask you to sign a consent form to indicate that you treatment. are happy for the procedure to go ahead. Before any further procedures we will again check that you are happy to proceed. How do we perform Plasma Exchange? What is a plasma exchange? Plasma exchange is performed using a machine Blood is made up of red cells, white cells and called a Blood Cell Separator which can separate platelets which are carried around in fluid called blood into its various parts. The machine separates plasma.