Case Reports
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Factors Affecting Mobilization of Peripheral Blood Progenitor Cells in Patients with Lymphoma’
Vol. 4, 311-316, February 1998 Clinical Cancer Research 311 Factors Affecting Mobilization of Peripheral Blood Progenitor Cells in Patients with Lymphoma’ Craig H. Moskowitz,2 Jill R. Glassman, (median, 13 versus 22 days; P 0.06). Patients who received 1l cycles of chemotherapy prior to PBPC mobilization David Wuest, Peter Maslak, Lilian Reich, tended to have delayed platelet recovery to >20,090/&l and Anthony Gucciardo, Nancy Coady-Lyons, to require more platelet transfusions than less extensively Andrew D. Zelenetz, and Stephen D. Nimer pretreated patients (median, 13.5 versus 23.5 days; P 0.15; Division of Hematologic Oncology, Department of Medicine median number of platelet transfusion episodes, 13 versus 9; [C. H. M., D. W., P. M., L. R., A. G., N. C-L., A. D. Z., S. D. N.] and P = 0.17). Department of Biostatistics [J. R. G.], Memorial Sloan-Kettering Cancer Center, New York, New York 10021 These data suggest that current strategies to mobilize PBPCs may be suboptimal in patients who have received either stem cell-toxic chemotherapy or 11 cycles of chem- ABSTRACT otherapy prior to PBPC mobilization. Alternative ap- The objective of this study was to identify factors asso- proaches, such as ex vivo expansion or the use of other ciated with poor mobilization of peripheral blood progenitor growth factors in addition to G-CSE, may improve mobili- cells (PBPCs) or delayed platelet engraftment after high- zation of progenitor cells for PBPC transplantation. dose therapy and autologous stem cell transplantation in patients with lymphoma. INTRODUCTION Fifty-eight patients with Hodgkin’s disease or non- The use of high-dose chemoradiotherapy supported by Hodgkin’s lymphoma underwent PBPC transplantation as cryopreserved autologous hematopoietic progenitor cells is ef- the “best available therapy” at Memorial Sloan-Kettering fective in treating relapsed HD3 and NHL; a high complete Cancer Center (New York, NY) between 1993 and 1995. -
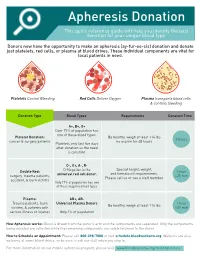
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

45 Part 606—Current Good Man- Ufacturing Practice
Food and Drug Administration, HHS Pt. 606 a presentation. The presiding officer ucts approved under § 601.91, the re- may, as a matter of discretion, permit strictions would no longer apply when questions to be submitted to the pre- FDA determines that safe use of the bi- siding officer for response by a person ological product can be ensured making a presentation. through appropriate labeling. FDA also (f) Judicial review. The Commissioner retains the discretion to remove spe- of Food and Drugs’ decision constitutes cific postapproval requirements upon final agency action from which the ap- review of a petition submitted by the plicant may petition for judicial re- sponsor in accordance with § 10.30 of view. Before requesting an order from a this chapter. court for a stay of action pending re- view, an applicant must first submit a PART 606—CURRENT GOOD MAN- petition for a stay of action under § 10.35 of this chapter. UFACTURING PRACTICE FOR BLOOD AND BLOOD COMPO- [67 FR 37996, May 31, 2002, as amended at 70 NENTS FR 14984, Mar. 24, 2005] § 601.93 Postmarketing safety report- Subpart A—General Provisions ing. Sec. Biological products approved under 606.3 Definitions. this subpart are subject to the post- marketing recordkeeping and safety Subpart B—Organization and Personnel reporting applicable to all approved bi- ological products. 606.20 Personnel. § 601.94 Promotional materials. Subpart C—Plant and Facilities For biological products being consid- 606.40 Facilities. ered for approval under this subpart, unless otherwise informed by the agen- Subpart D—Equipment cy, applicants must submit to the agency for consideration during the 606.60 Equipment. -

0985.03CC Non-Mobilized Donor Consent
Fred Hutchinson Cancer Research Center Consent to take part in a research study: 985.03CC – Non-Mobilized Donor Consent to Participate as a Donor of Non-Mobilized Peripheral Blood Mononuclear Cells for Laboratory Research and Process Development Studies. Principal Investigator: Derek Stirewalt, MD, Member, FHCRC, (206 667-5386) Investigators: Michael Linenberger, M.D., FACP Professor, Division of Hematology, UW, Robert & Phyllis Henigson Endowed Chair, Program Director, Hematology/Oncology Fellowship Medical Director, Apheresis and Cellular Therapy, SCCA, Member, FHCRC, Associate Professor of Medicine, UW, (206 667-5021); Laura Connelly Smith, Assistant Member, FHCRC, Assistant Professor, UW (206 606 -6938) Research Coordinator: Aubrey McMillan, (206) 667-3539 Emergency Phone (24 hours) UW Transplant unit 8NE: (206) 598-8902 UW Nocturnist on call provider (7:00 PM -7:00 AM): (206) 598-1062 Donor recruitment and participation: 206-667-5318 Important things to know about this study. You are invited to participate in a research study. The purpose of this research is to collect blood cells by a procedure called “leukapheresis”. Leukapheresis is a procedure that allows for a greater collection of peripheral blood cells than can be obtained by standard blood donation. Our purpose is to collect both small blood draws and larger samples of peripheral blood cells for laboratory research studies. This research may involve analysis of your genetic material called DNA and RNA. The studies may also examine other components of the cell like proteins. These analyses can be used to better understand cancer and other diseases. People who agree to join the study will be asked to attend 2 appointments over up to 30 days. -

Leukapheresis Protocol for Nonhuman Primates Weighing Less Than 10 Kg
Journal of the American Association for Laboratory Animal Science Vol 52, No 1 Copyright 2013 January 2013 by the American Association for Laboratory Animal Science Pages 70–77 Leukapheresis Protocol for Nonhuman Primates Weighing Less than 10 Kg Vimukthi Pathiraja, Abraham J Matar, Ashley Gusha, Christene A Huang, and Raimon Duran-Struuck* Leukapheresis is a common procedure for hematopoietic cell transplantation in adults. The main challenge in applying this procedure to human infants and small monkeys is the large extracorporeal blood volume (165 mL on average) necessary for priming the apheresis machine. This volume represents greater than 50% of the total circulating blood volume of a human neonate or small monkey. In this report, we document a safe leukapheresis protocol developed for rhesus macaques (3.9 to 8.7 kg). To avoid sensitizing donor animals undergoing leukapheresis to third-party blood products, autologous blood col- lected during the weeks prior to leukapheresis was used to volume-expand the same donor while priming the machine with saline on the day of leukapheresis. During the procedures, blood pressure was controlled by monitoring the inlet volume, and critical-care support was provided by the anesthesia team. Electrolytes and hemogram parameters were monitored intermit- tently. Overall, our research subjects underwent effective 4- to 6-h leukapheresis. A total of 9 leukapheresis procedures were performed, which yielded 1 × 109 to 6 × 109 peripheral blood mononuclear cells containing 1.1 to 5.1 × 106 CD34+ cells (assessed in 4 of 9 macaques) in a volume of 30 to 85 mL. All macaques showed decreases in Hct and platelet counts. -

Current Challenges in Providing Good Leukapheresis Products for Manufacturing of CAR-T Cells for Patients with Relapsed/Refractory NHL Or ALL
cells Article Current Challenges in Providing Good Leukapheresis Products for Manufacturing of CAR-T Cells for Patients with Relapsed/Refractory NHL or ALL Felix Korell 1,*, Sascha Laier 2, Sandra Sauer 1, Kaya Veelken 1, Hannah Hennemann 1, Maria-Luisa Schubert 1, Tim Sauer 1, Petra Pavel 2, Carsten Mueller-Tidow 1, Peter Dreger 1, Michael Schmitt 1 and Anita Schmitt 1 1 Department of Internal Medicine V, University Hospital Heidelberg, 69120 Heidelberg, Germany; [email protected] (S.S.); [email protected] (K.V.); [email protected] (H.H.); [email protected] (M.-L.S.); [email protected] (T.S.); [email protected] (C.M.-T.); [email protected] (P.D.); [email protected] (M.S.); [email protected] (A.S.) 2 Institute of Clinical Transfusion Medicine and Cell Therapy (IKTZ), 89081 Heidelberg, Germany; [email protected] (S.L.); [email protected] (P.P.) * Correspondence: [email protected] Received: 9 April 2020; Accepted: 13 May 2020; Published: 15 May 2020 Abstract: Background: T lymphocyte collection through leukapheresis is an essential step for chimeric antigen receptor T (CAR-T) cell therapy. Timing of apheresis is challenging in heavily pretreated patients who suffer from rapid progressive disease and receive T cell impairing medication. Methods: A total of 75 unstimulated leukaphereses were analyzed including 45 aphereses in patients and 30 in healthy donors. Thereof, 41 adult patients with Non-Hodgkin’s lymphoma (85%) or acute lymphoblastic leukemia (15%) underwent leukapheresis for CAR-T cell production. -

Cord Blood Stem Cell Transplantation
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights • Umbilical cord blood, like bone marrow and peripheral blood, is a rich source of stem cells for transplantation. There may be advantages for certain patients to have cord blood stem cell transplants instead of transplants with marrow or peripheral blood stem cells (PBSCs). • Stem cell transplants (peripheral blood, marrow or cord blood) may use the patient’s own stem cells (called “autologous transplants”) or use donor stem cells. Donor cells may come from either a related or unrelated matched donor (called an “allogeneic transplant”). Most transplant physicians would not want to use a baby’s own cord blood (“autologous transplant”) to treat his or her leukemia. This is because donor stem cells might better fight the leukemia than the child’s own stem cells. • Cord blood for transplantation is collected from the umbilical cord and placenta after a baby is delivered. Donated cord blood that meets requirements is frozen and stored at a cord blood bank for future use. • The American Academy of Pediatrics’s (AAP) policy statement (Pediatrics; 2007;119:165-170.) addresses public and private banking options available to parents. Among several recommendations, the report encourages parents to donate to public cord blood banks and discourages parents from using private cord blood banks for personal or family cord blood storage unless they have an older child with a condition that could benefit from transplantation. • The Stem Cell Therapeutic and Research Act of 2005 put several programs in place, including creation of the National Cord Blood Inventory (NCBI) for patients in need of transplantation. -

Therapeutic Apheresis, J Clin Apheresis 2007, 22, 104-105
Apheresis: Basic Principles, Practical Considerations and Clinical Applications Joseph Schwartz, MD Anand Padmanabhan, MD PhD Director, Transfusion Medicine Assoc Med Director/Asst Prof Columbia Univ. Medical Center BloodCenter of Wisconsin New York Presbyterian Hospital Medical College of Wisconsin Review Session, ASFA Annual meeting, Scottsdale, Arizona, June 2011 Objectives (Part 1) • Mechanism of Action • Definitions • Technology (ies) • Use • Practical Considerations • Math • Clinical applications – HPC Collection Objectives (Part 2) • Clinical applications: System/ Disease Specific Indications • ASFA Fact Sheet Apheresis •Derives from Greek, “to carry away” •A technique in which whole blood is taken and separated extracorporealy, separating the portion desired from the remaining blood. •This allows the desired portion (e.g., plasma) to be removed and the reminder returned. Apheresis- Mechanism of Action •Large-bore intravenous catheter connected to a spinning centrifuge bowl •Whole blood is drawn from donor/patient into the centrifuge bowl •The more dense elements, namely the RBC, settle to the bottom with less dense elements such as WBC and platelets overlying the RBC layer and finally, plasma at the very top. Apheresis: Principles of Separation Platelets (1040) Lymphocytes Torloni MD (1050-1061) Monocytes (1065 - 1069) Granulocyte (1087 - 1092) RBC Torloni MD Torloni MD Separate blood components is based on density with removal of the desired component Graphics owned by and courtesy of Gambro BCT Principals of Apheresis WBC Plasma Torlo RBC ni MD Torloni MD RBC WBC Plasma G Cobe Spectra Apheresis- Mechanism of Action Definitions • Plasmapheresis: plasma is separated, removed (i.e. less than 15% of total plasma volume) without the use of replacement solution • Plasma exchange (TPE): plasma is separated, removed and replaced with a replacement solution such as colloid (e.g. -

Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods
Guidance for Industry Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods Additional copies of this guidance document are available from: Office of Communication, Training and Manufacturers Assistance (HFM-40) 1401 Rockville Pike, Rockville, MD 20852-1448 (Tel) 1-800-835-4709 or 301-827-1800 (Internet) http://www.fda.gov/cber/guidelines.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research (CBER) January 2001 Technical Correction February 2001 TABLE OF CONTENTS Note: Page numbering may vary for documents distributed electronically. I. INTRODUCTION ............................................................................................................. 1 II. BACKGROUND................................................................................................................ 1 III. CHANGES FROM THE DRAFT GUIDANCE .............................................................. 2 IV. RECOMMENDED DONOR SELECTION CRITERIA FOR THE AUTOMATED RED BLOOD CELL COLLECTION PROTOCOLS ..................................................... 3 V. RECOMMENDED RED BLOOD CELL PRODUCT QUALITY CONTROL............ 5 VI. REGISTRATION AND LICENSING PROCEDURES FOR THE MANUFACTURE OF RED BLOOD CELLS COLLECTED BY AUTOMATED METHODS.................. 7 VII. ADDITIONAL REQUIREMENTS.................................................................................. 9 i GUIDANCE FOR INDUSTRY Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods This -

Principles of Blood Separation and Apheresis Instrumentation
Principles of Blood Separation and Apheresis Instrumentation Dobri Kiprov, M.D., H.P. Chief, Division of Immunotherapy, California Pacific Medical Center, San Francisco, CA Medical Director, Apheresis Care Group Apheresis History Apheresis History Apheresis History Apheresis From the Greek - “to take away” Blood separation Donor apheresis Therapeutic apheresis Principles of Blood Separation Filtration Centrifugation Combined centrifugation and filtration Membrane Separation Blood is pumped through a membrane with pores allowing plasma to pass through whilst retaining blood cells. Available as a hollow fiber membrane (older devices used parallel-plate membranes) Pore diameter for plasma separation: 0.2 to 0.6μm. A number of parameters need to be closely controlled Detail of Membrane Separation Courtesy of CaridianBCT Membrane Blood Separation Trans Membrane Pressure (TMP) Too High = Hemolysis TMP Too Low = No Separation Optimal TMP = Good Separation Membrane Apheresis in the US - PrismaFlex (Gambro – Baxter) - NxStage - BBraun Filtration vs. Centrifugation Apheresis Filtration Centrifugation Minimal availability The standard in the in the USA USA • Poor industry support • Very good industry support Limited to plasma Multiple procedures (cytapheresis) exchange • Opportunity to provide • Low efficiency cellular therapies Centrifugation vs. Filtration Apheresis Centrifugation Apheresis Filtration Apheresis Blood Flow 10 – 100 ml/min 150 ml/min Efficiency of Plasma 60 – 65% 30% Removal Apheresis in Clinical Practice and Blood Banking Sickle Cell Disease Falciparum Malaria Thrombocytosis RBC WBC PLT Plasma Leukemias TTP-HUS Cell Therapies Guillain Barre Syndrome Myasthenia Gravis CIDP Autoimmune Renal Disease Hyperviscosity Syndromes Centrifugal Separation Based on the different specific gravity of the blood components. In some instruments, also based on the cellular size (Elutriation). -

Transfusion Reactions 7
HEMATOLOGY Immunohematology, transfusion medicine and bone marrow transplantation Institute of Pathological Physiology First Facultry of Medicine, Charles University in Prague http://patf.lf1.cuni.cz Questions and Comments: MUDr. Pavel Klener, Ph.D., [email protected] Presentation in points 1. Imunohematology 2. AB0 blood group system 3. Rh blood group system 4. Hemolytic disease of the newborn and neonatal alloimmune thrombocytopenia 5. Pre-transfusion examinations 6. Transfusion reactions 7. Transfusion medicine and hemapheresis 8. HLA system 9. Stem cell/ bone marrow transplantation Origins of immunohematology and transfusion medicine Imunohematology as a branch of medicine developed hand in hand with the origins of transfusion therapy. It focuses on concepts and questions associated with transfusion therapy, immunisation (as a result of transfusion therapy and pregnancy) and organ transplantation. In 1665, an English physiologist, Richard Lower, successfully performed the first animal- to-animal blood transfusion that kept ex-sanguinated dogs alive by transfusion of blood from other dogs. In 1667, Jean Bapiste Denys, transfused blood from the carotid artery of a lamb into the vein of a young man, which at first seemed successful. However, after the third transfusion of lamb’s blood the man suffered a reaction and died. Due to the many disastrous consequences resulting from blood transfusion, transfusions were prohibited from 1667 to 1818- when James Blundell of England successfully transfused human blood to women suffering from hemorrhage at childbirth. Revolution in 1900 In 1900 Karl Landsteiner discovered the AB0 blood groups. This landmark event initiated the era of scientific – based transfusion therapy and was the foundation of immunohematology as a science.