Plateletpheresis: a Comparative Study Between Haemonetics MCS Plus and Spectra Trima
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unintentional Platelet Removal by Plasmapheresis
Journal of Clinical Apheresis 16:55–60 (2001) Unintentional Platelet Removal by Plasmapheresis Jedidiah J. Perdue,1 Linda K. Chandler,2 Sara K. Vesely,1 Deanna S. Duvall,2 Ronald O. Gilcher,2 James W. Smith,2 and James N. George1* 1Hematology-Oncology Section, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma 2Oklahoma Blood Institute, Oklahoma City, Oklahoma Therapeutic plasmapheresis may remove platelets as well as plasma. Unintentional platelet loss, if not recognized, may lead to inappropriate patient assessment and treatment. A patient with thrombotic thrombocytopenic purpura- hemolytic uremic syndrome (TTP-HUS) is reported in whom persistent thrombocytopenia was interpreted as continuing active disease; thrombocytopenia resolved only after plasma exchange treatments were stopped. This observation prompted a systematic study of platelet loss with plasmapheresis. Data are reported on platelet loss during 432 apheresis procedures in 71 patients with six disease categories using three different instruments. Com- paring the first procedure recorded for each patient, there was a significant difference among instrument types ,than with the COBE Spectra (1.6% (21 ס P<0.001); platelet loss was greater with the Fresenius AS 104 (17.5%, N) .With all procedures, platelet loss ranged from 0 to 71% .(24 ס or the Haemonetics LN9000 (2.6%, N (26 ס N Among disease categories, platelet loss was greater in patients with dysproteinemias who were treated for hyper- viscosity symptoms. Absolute platelet loss with the first recorded apheresis procedure, in the 34 patients who had a normal platelet count before the procedure, was also greater with the AS 104 (2.23 × 1011 platelets) than with the Spectra (0.29 × 1011 platelets) or the LN9000 (0.37 × 1011 platelets). -

Platelet-Rich Plasmapheresis: a Meta-Analysis of Clinical Outcomes and Costs
THE jOURNAL OF EXTRA-CORPOREAL TECHNOLOGY Original Article Platelet-Rich Plasmapheresis: A Meta-Analysis of Clinical Outcomes and Costs Chris Brown Mahoney , PhD Industrial Relations Center, Carlson School of Management, University of Minnesota, Minneapolis, MN Keywords: platelet-rich plasmapheresis, sequestration, cardiopulmonary bypass, outcomes, economics, meta-analysis Presented at the American Society of Extra-Corporeal Technology 35th International Conference, April 3-6, 1997, Phoenix, Arizona ABSTRACT Platelet-rich plasmapheresis (PRP) just prior to cardiopulmonary bypass (CPB) surgery is used to improve post CPB hemostasis and to minimize the risks associated with exposure to allogeneic blood and its components. Meta-analysis examines evidence ofPRP's impact on clinical outcomes by integrating the results across published research studies. Data on clinical outcomes was collected from 20 pub lished studies. These outcomes, DRG payment rates, and current national average costs were used to examine the impact of PRP on costs. This study provides evidence that the use of PRP results in improved clinical outcomes when compared to the identical control groups not receiving PRP. These improved clinical out comes result in subsequent lower costs per patient in the PRP groups. All clinical outcomes analyzed were improved: blood product usage, length of stay, intensive care stay, time to extu bation, incidence of cardiovascular accident, and incidence of reoperation. The most striking differences occur in use of all blood products, particularly packed red blood cells. This study provides an example of how initial expenditure on technology used during CPB results in overall cost savings. Estimated cost savings range from $2,505.00 to $4,209.00. -
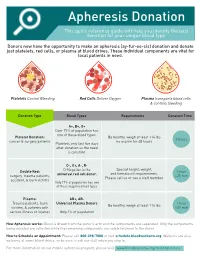
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Important Information for Female Platelet Donors
Important Information for Female Platelet Donors We are grateful for the support you provide our community blood program and especially appreciate your willingness to help save lives as a volunteer platelet donor. We also take our responsibility to provide a safe and adequate blood supply very seriously and need to share the following information regarding a change to our donor eligibility criteria for female platelet donors. We recently began performing a Human Leukocyte Antigen (HLA) antibody test on each of our current female platelet donors who have ever been pregnant. In addition, we modified our Medical History Questionnaire to ask donors whether they have been pregnant since their last donation. Platelet donors who respond yes to that question will be screened for HLA antibodies. Platelet donors will also be retested after every subsequent pregnancy. These adjustments are being made as part of our effort to reduce occurrences of Transfusion-Related Acute Lung Injury (TRALI). TRALI is a rare but serious complication of blood transfusions most commonly thought to be caused by a reaction to HLA antibodies present in the donor’s plasma. When transfused, these antibodies can sometimes cause plasma to leak into the patient’s lungs, creating fluid accumulation — a condition referred to as acute pulmonary edema. Female donors who have been pregnant are more likely than others to have these HLA antibodies in their plasma. Once the antibodies develop, they are present forever. The antibodies could be harmful if transfused into certain patients. The antibodies are present in plasma — and platelet donations contain a high volume of plasma, so our current efforts are directed at screening blood samples from female platelet donors to test for the HLA antibody. -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses. -

Cord Blood Stem Cell Transplantation
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights • Umbilical cord blood, like bone marrow and peripheral blood, is a rich source of stem cells for transplantation. There may be advantages for certain patients to have cord blood stem cell transplants instead of transplants with marrow or peripheral blood stem cells (PBSCs). • Stem cell transplants (peripheral blood, marrow or cord blood) may use the patient’s own stem cells (called “autologous transplants”) or use donor stem cells. Donor cells may come from either a related or unrelated matched donor (called an “allogeneic transplant”). Most transplant physicians would not want to use a baby’s own cord blood (“autologous transplant”) to treat his or her leukemia. This is because donor stem cells might better fight the leukemia than the child’s own stem cells. • Cord blood for transplantation is collected from the umbilical cord and placenta after a baby is delivered. Donated cord blood that meets requirements is frozen and stored at a cord blood bank for future use. • The American Academy of Pediatrics’s (AAP) policy statement (Pediatrics; 2007;119:165-170.) addresses public and private banking options available to parents. Among several recommendations, the report encourages parents to donate to public cord blood banks and discourages parents from using private cord blood banks for personal or family cord blood storage unless they have an older child with a condition that could benefit from transplantation. • The Stem Cell Therapeutic and Research Act of 2005 put several programs in place, including creation of the National Cord Blood Inventory (NCBI) for patients in need of transplantation. -

Therapeutic Apheresis, J Clin Apheresis 2007, 22, 104-105
Apheresis: Basic Principles, Practical Considerations and Clinical Applications Joseph Schwartz, MD Anand Padmanabhan, MD PhD Director, Transfusion Medicine Assoc Med Director/Asst Prof Columbia Univ. Medical Center BloodCenter of Wisconsin New York Presbyterian Hospital Medical College of Wisconsin Review Session, ASFA Annual meeting, Scottsdale, Arizona, June 2011 Objectives (Part 1) • Mechanism of Action • Definitions • Technology (ies) • Use • Practical Considerations • Math • Clinical applications – HPC Collection Objectives (Part 2) • Clinical applications: System/ Disease Specific Indications • ASFA Fact Sheet Apheresis •Derives from Greek, “to carry away” •A technique in which whole blood is taken and separated extracorporealy, separating the portion desired from the remaining blood. •This allows the desired portion (e.g., plasma) to be removed and the reminder returned. Apheresis- Mechanism of Action •Large-bore intravenous catheter connected to a spinning centrifuge bowl •Whole blood is drawn from donor/patient into the centrifuge bowl •The more dense elements, namely the RBC, settle to the bottom with less dense elements such as WBC and platelets overlying the RBC layer and finally, plasma at the very top. Apheresis: Principles of Separation Platelets (1040) Lymphocytes Torloni MD (1050-1061) Monocytes (1065 - 1069) Granulocyte (1087 - 1092) RBC Torloni MD Torloni MD Separate blood components is based on density with removal of the desired component Graphics owned by and courtesy of Gambro BCT Principals of Apheresis WBC Plasma Torlo RBC ni MD Torloni MD RBC WBC Plasma G Cobe Spectra Apheresis- Mechanism of Action Definitions • Plasmapheresis: plasma is separated, removed (i.e. less than 15% of total plasma volume) without the use of replacement solution • Plasma exchange (TPE): plasma is separated, removed and replaced with a replacement solution such as colloid (e.g. -

Essential Thrombocythemia Facts No
Essential Thrombocythemia Facts No. 12 in a series providing the latest information for patients, caregivers and healthcare professionals www.LLS.org • Information Specialist: 800.955.4572 Introduction Highlights Essential thrombocythemia (ET) is one of several l Essential thrombocythemia (ET) is one of a related “myeloproliferative neoplasms” (MPNs), a group of closely group of blood cancers known as “myeloproliferative related blood cancers that share several features, notably the neoplasms” (MPNs) in which cells in the bone “clonal” overproduction of one or more blood cell lines. marrow that produce the blood cells develop and All clonal disorders begin with one or more changes function abnormally. (mutations) to the DNA in a single cell; the altered cells in l ET begins with one or more acquired changes the marrow and the blood are the offspring of that one (mutations) to the DNA of a single blood-forming mutant cell. Other MPNs include polycythemia vera and cell. This results in the overproduction of blood cells, myelofibrosis. especially platelets, in the bone marrow. The effects of ET result from uncontrolled blood cell l About half of individuals with ET have a mutation production, notably of platelets. Because the disease arises of the JAK2 (Janus kinase 2) gene. The role that this from a change to an early blood-forming cell that has the mutation plays in the development of the disease, capacity to form red cells, white cells and platelets, any and the potential implications for new treatments, combination of these three cell lines may be affected – and are being investigated. usually each cell line is affected to some degree. -

Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods
Guidance for Industry Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods Additional copies of this guidance document are available from: Office of Communication, Training and Manufacturers Assistance (HFM-40) 1401 Rockville Pike, Rockville, MD 20852-1448 (Tel) 1-800-835-4709 or 301-827-1800 (Internet) http://www.fda.gov/cber/guidelines.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research (CBER) January 2001 Technical Correction February 2001 TABLE OF CONTENTS Note: Page numbering may vary for documents distributed electronically. I. INTRODUCTION ............................................................................................................. 1 II. BACKGROUND................................................................................................................ 1 III. CHANGES FROM THE DRAFT GUIDANCE .............................................................. 2 IV. RECOMMENDED DONOR SELECTION CRITERIA FOR THE AUTOMATED RED BLOOD CELL COLLECTION PROTOCOLS ..................................................... 3 V. RECOMMENDED RED BLOOD CELL PRODUCT QUALITY CONTROL............ 5 VI. REGISTRATION AND LICENSING PROCEDURES FOR THE MANUFACTURE OF RED BLOOD CELLS COLLECTED BY AUTOMATED METHODS.................. 7 VII. ADDITIONAL REQUIREMENTS.................................................................................. 9 i GUIDANCE FOR INDUSTRY Recommendations for Collecting Red Blood Cells by Automated Apheresis Methods This -

Principles of Blood Separation and Apheresis Instrumentation
Principles of Blood Separation and Apheresis Instrumentation Dobri Kiprov, M.D., H.P. Chief, Division of Immunotherapy, California Pacific Medical Center, San Francisco, CA Medical Director, Apheresis Care Group Apheresis History Apheresis History Apheresis History Apheresis From the Greek - “to take away” Blood separation Donor apheresis Therapeutic apheresis Principles of Blood Separation Filtration Centrifugation Combined centrifugation and filtration Membrane Separation Blood is pumped through a membrane with pores allowing plasma to pass through whilst retaining blood cells. Available as a hollow fiber membrane (older devices used parallel-plate membranes) Pore diameter for plasma separation: 0.2 to 0.6μm. A number of parameters need to be closely controlled Detail of Membrane Separation Courtesy of CaridianBCT Membrane Blood Separation Trans Membrane Pressure (TMP) Too High = Hemolysis TMP Too Low = No Separation Optimal TMP = Good Separation Membrane Apheresis in the US - PrismaFlex (Gambro – Baxter) - NxStage - BBraun Filtration vs. Centrifugation Apheresis Filtration Centrifugation Minimal availability The standard in the in the USA USA • Poor industry support • Very good industry support Limited to plasma Multiple procedures (cytapheresis) exchange • Opportunity to provide • Low efficiency cellular therapies Centrifugation vs. Filtration Apheresis Centrifugation Apheresis Filtration Apheresis Blood Flow 10 – 100 ml/min 150 ml/min Efficiency of Plasma 60 – 65% 30% Removal Apheresis in Clinical Practice and Blood Banking Sickle Cell Disease Falciparum Malaria Thrombocytosis RBC WBC PLT Plasma Leukemias TTP-HUS Cell Therapies Guillain Barre Syndrome Myasthenia Gravis CIDP Autoimmune Renal Disease Hyperviscosity Syndromes Centrifugal Separation Based on the different specific gravity of the blood components. In some instruments, also based on the cellular size (Elutriation). -

Transfusion Reactions 7
HEMATOLOGY Immunohematology, transfusion medicine and bone marrow transplantation Institute of Pathological Physiology First Facultry of Medicine, Charles University in Prague http://patf.lf1.cuni.cz Questions and Comments: MUDr. Pavel Klener, Ph.D., [email protected] Presentation in points 1. Imunohematology 2. AB0 blood group system 3. Rh blood group system 4. Hemolytic disease of the newborn and neonatal alloimmune thrombocytopenia 5. Pre-transfusion examinations 6. Transfusion reactions 7. Transfusion medicine and hemapheresis 8. HLA system 9. Stem cell/ bone marrow transplantation Origins of immunohematology and transfusion medicine Imunohematology as a branch of medicine developed hand in hand with the origins of transfusion therapy. It focuses on concepts and questions associated with transfusion therapy, immunisation (as a result of transfusion therapy and pregnancy) and organ transplantation. In 1665, an English physiologist, Richard Lower, successfully performed the first animal- to-animal blood transfusion that kept ex-sanguinated dogs alive by transfusion of blood from other dogs. In 1667, Jean Bapiste Denys, transfused blood from the carotid artery of a lamb into the vein of a young man, which at first seemed successful. However, after the third transfusion of lamb’s blood the man suffered a reaction and died. Due to the many disastrous consequences resulting from blood transfusion, transfusions were prohibited from 1667 to 1818- when James Blundell of England successfully transfused human blood to women suffering from hemorrhage at childbirth. Revolution in 1900 In 1900 Karl Landsteiner discovered the AB0 blood groups. This landmark event initiated the era of scientific – based transfusion therapy and was the foundation of immunohematology as a science.