Blood and Immunity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updates in Mastocytosis
Updates in Mastocytosis Tryptase PD-L1 Tracy I. George, M.D. Professor of Pathology 1 Disclosure: Tracy George, M.D. Research Support / Grants None Stock/Equity (any amount) None Consulting Blueprint Medicines Novartis Employment ARUP Laboratories Speakers Bureau / Honoraria None Other None Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Mastocytosis symposium and consensus meeting on classification and diagnostic criteria for mastocytosis Boston, October 25-28, 2012 2008 WHO Classification Scheme for Myeloid Neoplasms Acute Myeloid Leukemia Chronic Myelomonocytic Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic Leukemia Myelodysplastic Syndromes MDS/MPN, unclassifiable Chronic Myelogenous Leukemia MDS/MPN Polycythemia Vera Essential Thrombocythemia Primary Myelofibrosis Myeloproliferative Neoplasms Chronic Neutrophilic Leukemia Chronic Eosinophilic Leukemia, NOS Hypereosinophilic Syndrome Mast Cell Disease MPNs, unclassifiable Myeloid or lymphoid neoplasms Myeloid neoplasms associated with PDGFRA rearrangement associated with eosinophilia and Myeloid neoplasms associated with PDGFRB abnormalities of PDGFRA, rearrangement PDGFRB, or FGFR1 Myeloid neoplasms associated with FGFR1 rearrangement (EMS) 2017 WHO Classification Scheme for Myeloid Neoplasms Chronic Myelomonocytic Leukemia Acute Myeloid Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic -

Digitalcommons@UNMC Agranulocytosis
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1935 Agranulocytosis Gordon A. Gunn University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Gunn, Gordon A., "Agranulocytosis" (1935). MD Theses. 386. https://digitalcommons.unmc.edu/mdtheses/386 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. AGRANULOOYTOSIS ,- Senior Thesis by GOrdon .M.. Gunn INTRODUCTION Fifteen years ago the medioal profession new nothing of the disease spoken of in this paper as agranulocytosis. Since Schultz, in 1922, gave an accurate description of a fulminat ing case, agranulocytosis has oomettoClOCo.'UPy more and more prominence in the medical field. Today, the literature is fairly teeming with accounts of isolated cases of all descriptions. Added to this a confus ing nomenclature, varied classifications, and heterogeneous forms of treatment; and the large question of whether it is a disease entity, a group of diseases, or only a symptom complex, and some idea may be garnered as to the progress made. Time is a most important factor in diagnosis of this disease, and the prognosis at best is grave. The treatment has gone through the maze of trials as that of any other new disease; there must be a cause and so there must be some specific treatment. -

White Blood Cells and Severe COVID-19: a Mendelian Randomization Study
Journal of Personalized Medicine Article White Blood Cells and Severe COVID-19: A Mendelian Randomization Study Yitang Sun 1 , Jingqi Zhou 1,2 and Kaixiong Ye 1,3,* 1 Department of Genetics, Franklin College of Arts and Sciences, University of Georgia, Athens, GA 30602, USA; [email protected] (Y.S.); [email protected] (J.Z.) 2 School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China 3 Institute of Bioinformatics, University of Georgia, Athens, GA 30602, USA * Correspondence: [email protected]; Tel.: +1-706-542-5898; Fax: +1-706-542-3910 Abstract: Increasing evidence shows that white blood cells are associated with the risk of coronavirus disease 2019 (COVID-19), but the direction and causality of this association are not clear. To evaluate the causal associations between various white blood cell traits and the COVID-19 susceptibility and severity, we conducted two-sample bidirectional Mendelian Randomization (MR) analyses with summary statistics from the largest and most recent genome-wide association studies. Our MR results indicated causal protective effects of higher basophil count, basophil percentage of white blood cells, and myeloid white blood cell count on severe COVID-19, with odds ratios (OR) per standard deviation increment of 0.75 (95% CI: 0.60–0.95), 0.70 (95% CI: 0.54–0.92), and 0.85 (95% CI: 0.73–0.98), respectively. Neither COVID-19 severity nor susceptibility was associated with white blood cell traits in our reverse MR results. Genetically predicted high basophil count, basophil percentage of white blood cells, and myeloid white blood cell count are associated with a lower risk of developing severe COVID-19. -

Leukocyte Adhesion Deficiency-I
Clinical Communications Leukocyte adhesion deficiency-I: A this article’s Online Repository at www.jaci-inpractice.org for a comprehensive review of all published comprehensive listing of publications and patient numbers by cases country and Table E2 for a comprehensive listing of references). Elena Almarza Novoa, PhDa,b,*, Per investigator assessment, 113 patients were considered to have severe LAD-I, 63 moderate, and 147 not classified. Sanchali Kasbekar, PharmDc,*, d e Neutrophil CD18 expression was reported for 265 cases and was Adrian J. Thrasher, MD, PhD , Donald B. Kohn, MD , less than 2% in 135 patients (51%) and 2% or more in 130 Julian Sevilla, MD, PhDf, Tony Nguyen, MBAg, patients (49%). Four patients with CD18 greater than or equal Jonathan D. Schwartz, MDc,z, and to 2% were considered to have severe LAD-I (CD18% range, Juan A. Bueren, PhDa,b,z 2.4-17.3). Sex information was available for 282 patients, of which 148 (52%) were males. Clinical Implications Age at presentation was reported for 146 cases. For 63 patients with CD18 less than 2%, median presentation was at age 1 Leukocyte adhesion deficiency-I (LAD-I) is a rare, serious month (range, 0.03-18 months); for 62 patients with CD18 of disorder with severity determined by defective CD18 2% or more, median presentation was at age 6 months (range, expression. LAD-I is characterized by umbilical 0.03-192 months). HSCT was performed for 125 patients; 198 complications, granulocytosis, and diverse infections. patients did not undergo HSCT. Severe LAD-I is frequently fatal before the age of 2 years Infections were described for 248 (77%) of the 323 cases; fi without allogeneic transplant. -

Late Effects Among Long-Term Survivors of Childhood Acute Leukemia in the Netherlands: a Dutch Childhood Leukemia Study Group Report
0031-3998/95/3805-0802$03.00/0 PEDIATRIC RESEARCH Vol. 38, No.5, 1995 Copyright © 1995 International Pediatric Research Foundation, Inc. Printed in U.S.A. Late Effects among Long-Term Survivors of Childhood Acute Leukemia in The Netherlands: A Dutch Childhood Leukemia Study Group Report A. VAN DER DOES-VAN DEN BERG, G. A. M. DE VAAN, J. F. VAN WEERDEN, K. HAHLEN, M. VAN WEEL-SIPMAN, AND A. J. P. VEERMAN Dutch Childhood Leukemia Study Group,' The Hague, The Netherlands A.8STRAC ' Late events and side effects are reported in 392 children cured urogenital, or gastrointestinal tract diseases or an increased vul of leukemia. They originated from 1193 consecutively newly nerability of the musculoskeletal system was found. However, diagnosed children between 1972 and 1982, in first continuous prolonged follow-up is necessary to study the full-scale late complete remission for at least 6 y after diagnosis, and were effects of cytostatic treatment and radiotherapy administered treated according to Dutch Childhood Leukemia Study Group during childhood. (Pediatr Res 38: 802-807, 1995) protocols (70%) or institutional protocols (30%), all including cranial irradiation for CNS prophylaxis. Data on late events (relapses, death in complete remission, and second malignancies) Abbreviations were collected prospectively after treatment; late side effects ALL, acute lymphocytic leukemia were retrospectively collected by a questionnaire, completed by ANLL, acute nonlymphocytic leukemia the responsible pediatrician. The event-free survival of the 6-y CCR, continuous first complete remission survivors at 15 y after diagnosis was 92% (±2%). Eight late DCLSG, Dutch Childhood Leukemia Study Group relapses and nine second malignancies were diagnosed, two EFS, event free survival children died in first complete remission of late toxicity of HR, high risk treatment, and one child died in a car accident. -

Educational Commentary – Blood Cell Id: Peripheral Blood Findings in a Case of Pelger Huët Anomaly
EDUCATIONAL COMMENTARY – BLOOD CELL ID: PERIPHERAL BLOOD FINDINGS IN A CASE OF PELGER HUËT ANOMALY Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits, click on Earn CE Credits under Continuing Education on the left side of the screen. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes On completion of this exercise, the participant should be able to • discuss the morphologic features of normal peripheral blood leukocytes; • describe characteristic morphologic findings in Pelger-Huët cells; and • differentiate Pelger-Huët cells from other neutrophils in a peripheral blood smear. Case History: A 30 year old female had a routine CBC performed as part of a physical examination. Her CBC results are as follows: WBC=5.9 x 109/L, RBC=4.53 x 1012/L, Hgb=13.6 g/dL, Hct=40%, MCV=88.3 fL, MCH=29.7 pg, MCHC=32.9 g/dL, Platelet=184 x 109/L. Introduction The images presented in this testing event represent normal white blood cells as well as several types of neutrophils that may be seen in the peripheral blood when a patient has the Pelger-Huët anomaly. -

Correlation of Blood Culture and Band Cell Ratio in Neonatal Septicaemia
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 13, Issue 3 Ver. VI. (Mar. 2014), PP 55-58 www.iosrjournals.org Correlation of blood culture and band cell ratio in neonatal septicaemia. Nautiyal S1., *Kataria V. K1., Pahuja V. K1., Jauhari S1., Roy R. C.,1 Aggarwal B.2 1Department of Microbiology, SGRRIM&HS and SMIH, Dehradun, Uttarakhand, India. 2Department of Paediatrics, SGRRIM&HS and SMIH, Dehradun, Uttarakhand, India. Abstract: Background: Neonatal sepsis is a clinical syndrome characterized by signs and symptoms of infection with or without accompanying bacteraemia in the first month of life. Incidence differs among hospitals depending on variety of factors. Blood culture is considered gold standard for the diagnosis, but does not give a rapid result. Hence, there is a need to look for a surrogate marker for diagnosing neonatal septicaemia. Material & Methods: 335 neonates were studied for clinically suspected septicaemia over a period of one year. Blood was cultured and organism identified biochemically. Parameters of subjects like EOS, LOS and Band cell counts were recorded. Results analysed statistically. Results: Male preponderance was observed. Majority of the cases had a normal vaginal delivery. 47.46% cases had early onset septicaemia. Meconium stained liquor was the predominant risk factor .Culture positivity was found to be 32.24% and 87.96% of them also had band cells percentage ranging from 0 to >25. Conclusion: Band cell count can be used as a surrogate marker for neonatal septicaemia. An upsurge of Candida species as a causative agent in Neonatal septicaemia has been observed. -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -
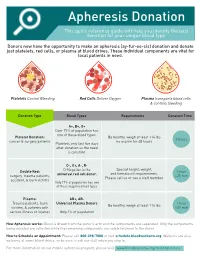
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

A Rapid and Quantitative D-Dimer Assay in Whole Blood and Plasma on the Point-Of-Care PATHFAST Analyzer ARTICLE in PRESS
MODEL 6 TR-03106; No of Pages 7 ARTICLE IN PRESS Thrombosis Research (2007) xx, xxx–xxx intl.elsevierhealth.com/journals/thre REGULAR ARTICLE A rapid and quantitative D-Dimer assay in whole blood and plasma on the point-of-care PATHFAST analyzer Teruko Fukuda a,⁎, Hidetoshi Kasai b, Takeo Kusano b, Chisato Shimazu b, Kazuo Kawasugi c, Yukihisa Miyazawa b a Department of Clinical Laboratory Medicine, Teikyo University School of Medical Technology, 2-11-1 Kaga, Itabashi, Tokyo 173-8605, Japan b Department of Central Clinical Laboratory, Teikyo University Hospital, Japan c Department of Internal Medicine, Teikyo University School of Medicine, Japan Received 11 July 2006; received in revised form 7 December 2006; accepted 28 December 2006 KEYWORDS Abstract The objective of this study was to evaluate the accuracy indices of the new D-Dimer; rapid and quantitative PATHFAST D-Dimer assay in patients with clinically suspected Deep-vein thrombosis; deep-vein thrombosis (DVT). Eighty two consecutive patients (34% DVT, 66% non-DVT) Point-of-care testing; with suspected DVT of a lower limb were tested with the D-Dimer assay with a Chemiluminescent PATHFAST analyzer. The diagnostic value of the PATHFAST D-Dimer assay (which is immunoassay based on the principle of a chemiluminescent enzyme immunoassay) for DVT was evaluated with pre-test clinical probability, compression ultrasonography (CUS). Furthermore, each patient underwent contrast venography and computed tomogra- phy, if necessary. The sensitivity and specificity of the D-Dimer assay using 0.570 μg/ mL FEU as a clinical cut-off value was found to be 100% and 63.2%, respectively, for the diagnosis of DVT, with a positive predictive value (PPV) and negative predictive value (NPV) of 66.7% and 100%, respectively. -

The Complete Blood Count Sample Report 1
THE COMPLETE BLOOD COUNT SAMPLE REPORT 1. Name and address of the lab where the test was performed. Tests may be run in a physician office lab, a lab located in a Different laboratories generate reports that can vary greatly in appearance and in the order and kind of The Complete Blood Count Sample Report clinic or hospital, and/or samples may be sent to a reference information included. This is one example of what a lab report for a Complete Blood Count may look like. laboratory for analysis. NamesDifferent and laboratories places used generate have been reports made that up can for vary illustrative greatly inpurposes appearance only. andThe innumbered the order keyand tokind the of right 2. Date this copy of the report was printed. This date may be explainsinformation a few included. of the reportThis is elements.one exampl e of what a lab report for a Complete Blood Count may look like. different than the date the results were generated, especially Names and places used have been made up for illustrative purposes only. Point your cursor at a number on cumulative reports (those that include results of several to learn about the different report elements. different tests run on different days). 3. Patient name or identifier. Links results to the correct person. 1 University Medical Center, Dept. of Pathology Report Date/Time: 123 University Way, City, ST 12345 02/10/2014 16:40 2 4. Patient identifier and identification number. Links results to the correct person. 3 Name: Doe, John Q. Age/Sex: 73/M DOB: 01/01/1941 5. -

Patient Information Leaflet – Plasma Exchange Procedure
Therapeutic Apheresis Services Patient Information Leaflet – Plasma Exchange Procedure Introduction Antibodies, which normally help to protect you from infection, can begin to attack your own This leaflet has been written to give patients healthy cells, or an over production of proteins can information about plasma exchange (sometimes cause your blood to become thicker and slow down called plasmapheresis). If you would like any more the blood flow throughout your body. A plasma information or have any questions, please ask the exchange can help improve your symptoms, doctors and nurses involved in your treatment at although this may not happen immediately. the NHS Blood and Transplant (NHSBT) Therapeutic Apheresis Services Unit. Although plasma exchange may help with symptoms, it will not normally cure your condition When you have considered the information given as it does not switch off the production of the in this leaflet, and after we have discussed the harmful antibodies or proteins. It is likely that procedure and its possible risks with you, we will this procedure will form only one part of your ask you to sign a consent form to indicate that you treatment. are happy for the procedure to go ahead. Before any further procedures we will again check that you are happy to proceed. How do we perform Plasma Exchange? What is a plasma exchange? Plasma exchange is performed using a machine Blood is made up of red cells, white cells and called a Blood Cell Separator which can separate platelets which are carried around in fluid called blood into its various parts. The machine separates plasma.