Platelet Product Administration Safety
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transfusion Service Introduction Blood/Blood Products Requests and Turnaround Time Expectations
Transfusion Service Introduction All blood products and blood components are supplied to UnityPoint Health-Meriter Hospital by the American Red Cross Blood Services. Pathology consultation is available regarding blood and/or components and dosages. ABO and Rho(D)—specific type is used whenever possible for leuko-poor packed cell transfusions. ABO-compatible blood is used for all plasma and platelet components whenever possible. For any orders involving HLA-matched components, the patient must have been HLA typed (sent to the American Red Cross) a minimum of 48 hours prior to intended infusion of the component. HLA typing is only required once. Blood components that are thawed, pooled, washed, volume-reduced, or deglycerolized for a patient will be charged to the patient even if not transfused. The charge is done because these components may not be suitable for another patient. Examples include: Autologous or directed donations Pooled cryoprecipitate 4-hour expiration Thawed fresh frozen plasma 24-hour expiration Thawed cryoprecipitate 6-hour expiration Deglycerolized red cells 24-hour expiration Washed red cells 24-hour expiration All blood components must be completely infused within 4 hours of release from UnityPoint Health-Meriter Laboratories Blood Bank, or be infused within the expiration time. Refer to UnityPoint Health -Meriter’s Blood and Blood Products Transfusion Policy #123 for additional information located on MyMeriter. Blood/Blood Products Requests and Turnaround Time Expectations Requests from UnityPoint Health-Meriter Hospital are entered in the hospital computer system and print in the UnityPoint Health- Meriter Laboratories Blood Bank. For the comfort of the patient, it is important to coordinate collection for other tests with Blood Bank specimens. -

Fresh Frozen Plasma in General Practice
Practical guide to using frozen and fresh frozen plasma in general practice Why every practice should keep a bag in the freezer Kit Sturgess; MA; VetMB; PhD; CertVR; DSAM; CertVC; FRCVS RCVS Recognised Specialist in Small Animal Medicine Advanced Practitioner in Veterinary Cardiology Introduction Keeping stock at reasonable levels in a practice is vital to good business management balancing the need to have a product available against the costs of keeping stock that is not used and potentially may go out of date and need to be replaced (as well as subtle small costs of space, stock taking, disposal of out-of-date product etc.). Practices need, therefore, to make decisions about preparedness for ‘what if’ scenarios and have protocols in place. The difficult question for many practices is how bizarre/uncommon should these scenarios be to warrant keeping a drug or treatment in stock or buying a specialist piece of equipment. This can only really be answered on an individual practice basis as it will depend on the type of cases seen as well as the likely ability of clients to want to pay if the treatment/investigation is expensive and the relationship with other local practices in terms of borrowing treatments or using equipment. It is also important to try and understand the value that a particular drug or treatment will have on morbidity and mortality of a particular condition and whether the patient could be referred onwards if necessary so not having calcium available if presented with a seizuring hypocalcaemic patient would be a significant issue in that patient’s care. -

Blood Banking/Transfusion Medicine Fellowship Program
DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE BLOOD BANKING/TRANSFUSION MEDICINE FELLOWSHIP PROGRAM THIS NEW ONE-YEAR ACGME-ACCREDITED FELLOWSHIP IN BLOOD BANKING/TRANSFUSION MEDICINE OFFERS STATE OF THE ART COMPREHENSIVE TRAINING IN BLOOD BANKING, COAGULATION, APHERESIS, AND HEMOTHERAPY AT THE MEMORIAL HERMANN HOSPITAL (MHH)-TEXAS MEDICAL CENTER (TMC) FOR PEDIATRICS AND ADULTS. This clinically-oriented fellowship is ideal for the candidate looking for exceptional experience in hemotherapy decision-making and coagulation consultation. We have a full spectrum of medical and surgical specialties, including a level 1 trauma center, as well as a busy solid organ transplant service (renal, liver, pancreas, cardiac, and lung). Fellows will rotate through: Our unique hemotherapy service: This innovative clinical consultative service for the Heart and Vascular Institute (HVI) allows fellows to serve as interventional blood banking consultants at the bed side as part of a multidisciplinary care team; our patients have complex bleeding and coagulopathy issues. Therapeutic apheresis service: This consultative service provide 24/7 direct patient care covering therapeutic plasmapheresis, red blood cell (RBC) exchanges, photopheresis, plateletpheresis, leukoreduction, therapeutic phlebotomies, and other related procedures on an inpatient and outpatient basis. We performed approximately over 1000 therapeutic plasma exchanges and RBC exchanges every year. Bloodbank: The MHH-TMC reference lab is one of the largest in Southeast Texas. This rotation provides extensive experience in interpreting antibody panel reports, working up transfusion reactions, investigating blood compatibility/incompatibility issues, and monitoring component usage. Gulf Coast Regional Blood Center: This rotation provides donor exposure in one of the largest community blood donation centers in the US as well as cellular therapy and immunohematology training. -
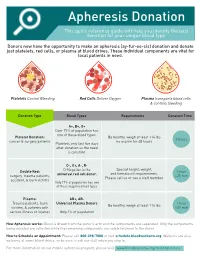
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Transfusion Medicine/Blood Banking
Transfusion Medicine/Blood Banking REQUIRED rotation This is a onetime rotation and is not planned by PGY level. Objective: The objective of this rotation is to teach fellows the clinical and laboratory aspects of Transfusion Medicine and Blood Banking as it impacts hematology/oncology. Goals: The goal of this rotation is to teach fellows blood ordering practices, the difference of type and screen and type and cross matches and selection of appropriate products for transfusion. Fellows will also learn to perform, watch type and screen red cell antibodies and will learn the significance of ABO, Rh and other blood group antigen systems briefly so as to understand the significance of red cell antibodies in transfusion practices. Fellows will learn the different types of Blood components, their indications and contraindications and component modification. e.g. Irradiated and washed products, and special product request and transfusions e.g. granulocyte transfusion, CMV negative products etc. Fellows will learn and become proficient in the laboratory aspects of autoimmune hemolytic anemia, coagulopathies and bleeding disorders in relation to massive transfusions and the laboratory aspects and management of platelet refractoriness. Fellows will learn some aspects of coagulation factor replacement for factor deficiencies and inhibitors. Activities: Fellows will rotate in the Blood Bank at UMC and learn to perform, observe and interpret standard blood bank procedures such as ABO Rh typing, Antibody Screen, Antibody Identification and Direct Antiglobulin Test (DAT). Fellows will also watch platelet cross matching for platelet refractoriness. Fellows will attend and present a few didactic lectures of about 40 minutes on Blood Bank related topics as they apply to hematology/oncology like blood group antigens, Blood Component therapy, Transfusion reactions, Autoimmune hemolytic anemia, Platelet immunology and HLA as it applies to transfusion medicine. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Important Information for Female Platelet Donors
Important Information for Female Platelet Donors We are grateful for the support you provide our community blood program and especially appreciate your willingness to help save lives as a volunteer platelet donor. We also take our responsibility to provide a safe and adequate blood supply very seriously and need to share the following information regarding a change to our donor eligibility criteria for female platelet donors. We recently began performing a Human Leukocyte Antigen (HLA) antibody test on each of our current female platelet donors who have ever been pregnant. In addition, we modified our Medical History Questionnaire to ask donors whether they have been pregnant since their last donation. Platelet donors who respond yes to that question will be screened for HLA antibodies. Platelet donors will also be retested after every subsequent pregnancy. These adjustments are being made as part of our effort to reduce occurrences of Transfusion-Related Acute Lung Injury (TRALI). TRALI is a rare but serious complication of blood transfusions most commonly thought to be caused by a reaction to HLA antibodies present in the donor’s plasma. When transfused, these antibodies can sometimes cause plasma to leak into the patient’s lungs, creating fluid accumulation — a condition referred to as acute pulmonary edema. Female donors who have been pregnant are more likely than others to have these HLA antibodies in their plasma. Once the antibodies develop, they are present forever. The antibodies could be harmful if transfused into certain patients. The antibodies are present in plasma — and platelet donations contain a high volume of plasma, so our current efforts are directed at screening blood samples from female platelet donors to test for the HLA antibody. -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses. -

Donor Sex, Age and Ethnicity Impact Stored Red Blood Cell Antioxidant Metabolism Through Mechanisms in Part Explained by Glucose
Blood Transfusion SUPPLEMENTARY APPENDIX Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity Angelo D’Alessandro, 1,2,3 Xiaoyun Fu, 4 Tamir Kanias, 3,5 Julie A. Reisz, 1 Rachel Culp-Hill, 1 Yuelong Guo, 6 Mark T. Gladwin, 5 Grier Page, 6 Steve Kleinman, 7 Marion Lanteri, 8 Mars Stone, 8 Michael P. Busch, 8# and James C. Zimring 9# for the Recipi - ent Epidemiology and Donor Evaluation Study-III (REDS III) 1Department of Biochemistry and Molecular Genetics, University of Colorado Denver – Anschutz Medical Campus, Aurora, CO, USA; 2De - partment of Medicine – Division of Hematology, University of Colorado Denver – Anschutz Medical Campus, Aurora, CO, USA; 3Vitalant Re - search Institute (previously Blood Systems Research Institute), Denver, CO, USA; 4Bloodworks Northwest Research Institute, Seattle, WA, USA; 5University of Pittsburgh, Pittsburgh, PA, USA; 6RTI International, Atlanta, GA, USA; 7University of British Columbia, Victoria, Canada; 8Vi - talant Research Institute (previously Blood Systems Research Institute), San Francisco, CA, USA and 9University of Virginia, Charlotesville, VA, USA #MPB and JCZ contributed equally as co-senior authors ©2021 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol. 2020.246603 Received: January 7, 2020. Accepted: March 27, 2020. Pre-published: April 2, 2020. Correspondence: ANGELO D’ALESSANDRO - [email protected] SUPPLEMENTARY MATERIAL -

Blood Establishment Registration and Product Listing .3 Change in Information
FORM APPROVED: OMB No. 0910-0052. Expiration Date: March 31, 2015. See page 3 for Burden Statement. 1. REGISTRATION NUMBER 3. REASON FOR SUBMISSION FOR FDA USE ONLY DEPARTMENT OF HEALTH AND HUMAN SERVICES FEI : .1 ANNUAL REGISTRATION FOOD AND DRUG ADMINISTRATION CFN : .2 INITIAL REGISTRATION 2. U.S. LICENSE NUMBER BLOOD ESTABLISHMENT REGISTRATION AND PRODUCT LISTING .3 CHANGE IN INFORMATION PLEASE READ INSTRUCTIONS CAREFULLY. Be sure to indicate any changes in your legal This form is authorized by Sections 510(b), (j) and 704 of the Federal Food, Drug, and Cosmetic Act (Title name or actual location in item 4, and any changes in your mailing address in item 6. Print all 21, United States Code 360(b), (j) and 374). Failure to report this information is a violation of Section 301(f) entries and make all corrections in red ink, if possible. Enter your phone number in item 8.3 and and (p) of the Act (Title 21, United States Code 331(f) and (p)) and can result in a fine of up to $1,000 or the phone number of your actual location in item 4.1. Sign the form and return to FDA. After imprisonment up to one year or both, pursuant to Section 303(a) of the Act (Title 21, United States Code validation, you will receive your Official Registration for the ensuing year. 33.3(a)). DISTRICT OFFICE: ENTER ALL CHANGES IN RED INK AND CIRCLE. 9. TYPE OF OWNERSHIP 10. TYPE ESTABLISHMENT (Check all boxes that describe routine or autologous operations.) 4. LEGAL NAME AND LOCATION (Include legal name, number and street, city, .1 SINGLE PROPRIETORSHIP .1 COMMUNITY (NON-HOSPITAL) BLOOD BANK state, country, and post office code.) .2 PARTNERSHIP .2 HOSPITAL BLOOD BANK .3 CORPORATION profit ___ non-profit ___ .3 PLASMAPHERESIS CENTER .4 COOPERATIVE ASSOCIATION .4 PRODUCT TESTING LABORATORY .5 FEDERAL (non-military) a. -

Cryoprecipitate
VUMC Blood Bank Website Products Page Cryoprecipitate Dosage: The VUMC blood bank maintains two distinct cryoprecipitate products. Adult patients will receive pre-pooled units of cryoprecipitate, these units contain 5 individual cryo units. Providers caring for adult patients can order pre-pooled cryoprecipitate in increments, with a traditional order of 2 pre-pooled cryoprecipitate units for an adult patient. Pediatric patients at VUMC receive cryoprecipitate via individual units according to weight based transfusion guidelines (recommended 10-15 mL/kg). Orders for cryoprecipitate that deviate from this algorithm - as well as orders for cryoprecipitate for patients without a recent fibrinogen level document in Starpanel - are flagged for review by the blood bank resident and/or the medical director. Introduction: According to standards set by the AABB, each unit of cryoprecipitate must contain at least 150 mg of fibrinogen. Cryoprecipitate also contains at least 80 IU of Factor VIII and appreciable amounts of von Willebrand Factor (vWF) and Factor XIII. Cryoprecipitate does not contain appreciable amounts of the other clotting factors. Indications: The most common indication for cryoprecipitate transfusion is hypofibrinogenemia, usually in the setting of DIC or major surgery but occasionally do to hereditary hypofibrinogenemia. Less commonly, cryoprecipitate has been used to provide factor replacement in Factor XIII deficiency. Please note, that human factor XIII concentrates are FDA approved for maintenance therapy (www.corifact.com). Cryoprecipitate should NOT be used for treatment of hemophilia A (Factor VIII deficiency) or von Willebrand’s disease. Vanderbilt discourages the use of cryoprecipitate as a post-surgical fibrin sealant. Special Information Unlike RBCs, platelets, and FFP, once cryoprecipitate is thawed, it cannot be re-stocked (re-frozen) by the blood bank. -

Cord Blood Stem Cell Transplantation
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights • Umbilical cord blood, like bone marrow and peripheral blood, is a rich source of stem cells for transplantation. There may be advantages for certain patients to have cord blood stem cell transplants instead of transplants with marrow or peripheral blood stem cells (PBSCs). • Stem cell transplants (peripheral blood, marrow or cord blood) may use the patient’s own stem cells (called “autologous transplants”) or use donor stem cells. Donor cells may come from either a related or unrelated matched donor (called an “allogeneic transplant”). Most transplant physicians would not want to use a baby’s own cord blood (“autologous transplant”) to treat his or her leukemia. This is because donor stem cells might better fight the leukemia than the child’s own stem cells. • Cord blood for transplantation is collected from the umbilical cord and placenta after a baby is delivered. Donated cord blood that meets requirements is frozen and stored at a cord blood bank for future use. • The American Academy of Pediatrics’s (AAP) policy statement (Pediatrics; 2007;119:165-170.) addresses public and private banking options available to parents. Among several recommendations, the report encourages parents to donate to public cord blood banks and discourages parents from using private cord blood banks for personal or family cord blood storage unless they have an older child with a condition that could benefit from transplantation. • The Stem Cell Therapeutic and Research Act of 2005 put several programs in place, including creation of the National Cord Blood Inventory (NCBI) for patients in need of transplantation.