Q and a About Pathogen Reduced Apheresis Platelet Components
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Transfusions
Guidelines for Transfu- sion Prepared by: Community Transfusion Committee Lincoln, Nebraska CHAIR: Aina Silenieks, MD MEMBERS: L. Bausch, M.D. R. Burton, M.D. S. Dunder, M.D. D. Voigt, M.D. B. J. Wilson, M.D. COMMUNITY Becky Croner REPRESENTATIVES: Ellen DiSalvo Christa Engel Phyllis Ericson Kelly Gillaspie Pat Gilles Vic Grdina Jessica Henrichs Kelly Jensen Laurel McReynolds Christina Nickel Angela Novotny Kelley Thiemann Janet Wachter Jodi Wikoff Guidelines For Transfusion Community Transfusion Committee INTRODUCTION The Community Transfusion Committee is a multidisciplinary group that meets to monitor blood utilization practices, establish guidelines for transfusion and discuss relevant transfusion related topics. It is comprised of physicians from local hospitals, invited guests, and community representatives from the hospitals’ transfusion services, nursing services, perfusion services, health information management, and the Nebraska Community Blood Bank. These Guidelines for Transfusion are reviewed and revised biannually by the Community Trans- fusion Committee to ensure that the industry’s most current practices are promoted. The Guidelines are the standard by which utilization practices are evaluated. They are also de- signed to provide helpful information to assist physicians to provide appropriate blood compo- nent therapy to patients. Appendices have been added for informational purposes and are not to be used as guidance for clinical decision making. ADULT RED CELLS A. Indications 1. One of the following a. Hypovolemia and hypoxia (signs/symptoms: syncope, dyspnea, postural hypoten- sion, tachycardia, angina, or TIA) secondary to surgery, trauma, GI tract bleeding, or intravascular hemolysis, OR b. Evidence of acute loss of 15% of total blood volume or >750 mL blood loss, OR c. -

Requirements for Blood and Blood Components Intended for Transfusion Or for Further Manufacturing Use
Requirements for Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use Office of Blood Research and Review CBER, FDA Ask the FDA Session – AABB Annual Meeting October 26, 2015 1 Outline • Overview – Historical Background – Intent of the Rule – Organization of the Rule • Selected Provisions – Relevant Transfusion-Transmitted Infection – Control of Bacterial Contamination of Platelets – Medical Supervision – Donor Acknowledgement – Alternative Procedures – Donor Eligibility • Implementation 2 GAO Oversight and FDA Concerns • Publish in the form of regulations the guidelines that FDA deems essential to ensure the safety of the blood supply • Concern about the delay in requiring testing for emerging infectious agents, e.g. HTLV • Concern about blood safety and the regulations being out-of-date • Concerns about donor safety 3 Intent of the Final Rule • To better assure the safety of the blood supply and to help protect donor health • To make donor eligibility and testing requirements more consistent with current practices and to provide flexibility with regard to emerging infectious diseases • To accommodate technological advances • To establish requirements for donor education, donor history, and donor testing 4 Organization of the Final Rule - 1 • A. General • B. Definitions (§§ 606.3, 610.39, 630.3, 640.125) • C. Standard Operating Procedures (§ 606.100) • D. Control of Bacterial Contamination of Platelets (§ 606.145) • E. Records (§ 606.160) • F. Test Requirements (§§ 610.40, 640.5, 640.71(a)) • G. Donor Deferral (§ 610.41) 5 Organization of the Final Rule - 2 • H. Purpose and Scope (§ 630.1) • I. Medical Supervision (§ 630.5) • J. General Donor Eligibility Requirements(§ 630.10) • K. Donor Eligibility Requirements Specific to Whole Blood, Red Blood Cells and Plasma Collected by Apheresis (§ 630.15) • L. -

27. Clinical Indications for Cryoprecipitate And
27. CLINICAL INDICATIONS FOR CRYOPRECIPITATE AND FIBRINOGEN CONCENTRATE Cryoprecipitate is indicated in the treatment of fibrinogen deficiency or dysfibrinogenaemia.1 Fibrinogen concentrate is licenced for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenaemia and hypofibrinogenaemia,2 and is currently funded under the National Blood Agreement. Key messages y Fibrinogen is an essential component of the coagulation system, due to its role in initial platelet aggregation and formation of a stable fibrin clot.3 y The decision to transfuse cryoprecipitate or fibrinogen concentrate to an individual patient should take into account the relative risks and benefits.3 y The routine use of cryoprecipitate or fibrinogen concentrate is not advised in medical or critically ill patients.2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In the setting of major obstetric haemorrhage, early administration of cryoprecipitate or fibrinogen concentrate may be necessary.3 Clinical implications y The routine use of cryoprecipitate or fibrinogen concentrate in medical or critically ill patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of disseminated intravascular coagulopathy (MED-PP18, CC-PP7).2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In patients with critical bleeding requiring massive transfusion, suggested doses of blood components is 3-4g (CBMT-PP10)3 in adults or as per the local Massive Transfusion Protocol. -

Platelet Transfusion
Lab Dept: Transfusion Services Test Name: PLATELET TRANSFUSION General Information Lab Order Codes: TPLT Special charges: HLA Matched-MHLA, AHLA PLA1 Negative – PLAT, APLA1 Platelet Crossmatching PLTX – CPLT Synonyms: Leukocyte Reduced Platelets; Random Platelets; Platelet Rich Plasma; Platelet concentrate; Leukocyte Reduced Platelet Pheresis; Single- Donor Platelets; SDP Platelet pheresis; Apheresis Platelets; LRPH CPT Codes: P9035 – Platelet pheresis, Leukocyte reduced 86806 – Lymphocytotoxicity assay, visual crossmatch, without titration 86813 – HLA Matched 86945 – Irradiation 86985 – Volume Reduction 86965 – Pooling 86903 – Platelet Antigen typing 86999 – Washing 86022 – Platelet Crossmatch Test Includes: Leukocyte Reduced Platelet Pheresis consists of platelets suspended in 200-300 mL of plasma collected by cytapheresis. Each unit contains at least 3 x 1011 platelets, and ≤5.0 x 106 leukocytes. One pheresis unit equals 5-6 random unit platelet concentrate. Logistics Test Indications: Refer to Guidelines for the Transfusion of Blood Components. Platelet Crossmatching or HLA-Matched Platelets may be useful for patients receiving repeated platelet transfusions who have become refractile, and for patients who repeatedly develop febrile non-hemolytic transfusion reactions after platelet transfusions. Volume Reduced Platelets are indicated in the event that ABO - incompatible platelets must be transfused due to availability or in patients with severe fluid restrictions. Refer to Blood Component Compatibility Chart Lab Testing Sections: Transfusion Services Phone Numbers: MIN Lab: 612-813-6824 STP Lab: 651-220-6558 Test Availability: Daily, 24 hours Turnaround Time: 1 - 2 hours Standard Dose/Volume: <10 kg: 10 – 15 mL/kg up to one unit or 50 mL maximum 10 – 15 kg: 1/3 pheresis unit 15 – 25 kg: 1/2 pheresis unit >25 kg: 1 pheresis unit Rate of Infusion: 10 minutes/unit, <4 hours Administration: Must be administered through a blood component administration filter. -
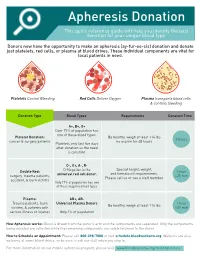
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Changes to the Technical Manual, 18Th Edition Monday, November 17, 2014 12:00 P.M
Changes to the Technical Manual, 18th Edition Monday, November 17, 2014 12:00 p.m. – 1:30 p.m. (ET) / 5:00p.m. – 6:30 p.m. (GMT) When this file is opened, Acrobat Reader will, by default, display the slides including the Acrobat reader controls. To return to full screen mode, hit Ctrl-L on your computer keyboard or use your mouse to click View>Full Screen on the menu bar of the Acrobat Reader program. To take the slides out of full screen mode and display the Acrobat Reader controls, simply hit the Esc key on your computer keyboard. To advance slides during the program, use the Enter, Page Down, down arrow or right arrow on your computer keyboard. To back up slides during the program, use the Page Up, up arrow of left arrow on your computer keyboard. Please remember to logon to the Live Learning Center using your email address and password to complete an evaluation of the program and speakers. At this time, advance to the next slide and wait for the audioconference to begin. A 2014 Audioconference presented to you by AABB The AABB Technical Manual 18th Edition What’s new? www.aabb.org Technical Manual by the Numbers • 1953 =year of first edition • 69 = number of authors/editors this edition • 370,378 = word count • 96 = number of methods • 60 = number of countries where TM is used www.aabb.org It’s a Process www.aabb.org Overview of Changes Major • Molecular testing • Patient blood management • Cellular therapy • Methods Minor • Throughout www.aabb.org 1: Quality Systems 2: Facilities and Safety • These 2 chapters are comprehensive discussions -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Guidance for the Provision of Intraoperative Cell Salvage
Enter Organisation details here GUIDANCE FOR THE PROVISION OF INTRAOPERATIVE CELL SALVAGE Guidance Guidance forfor Australian Australian Health Health Providers Providers MARCH> MARCH 2014 2014 Guidance for the provision of Intraoperative Cell Salvage Page 0 Ref No: Enter Organisation Ref AUS ICS Version No: 1 March 2014 Enter Organisation details here With the exception of any logos and registered trademarks, and where otherwise noted, all material presented in this document is provided under a Creative Commons Attribution 3.0 Australia (http://creativecommons.org/ licenses/by/3.0/au/) licence. The details of the relevant licence conditions are available on the Creative Commons website (accessible using the links provided) as is the full legal code for the CC BY 3.0 AU license (http://creativecommons.org/licenses/by/3.0/au/legalcode). The content obtained from this document or derivative of this work must be attributed as the Guidance for the provision of Intraoperative Cell Salvage. © National Blood Authority, 2014. ISBN 978-0-9873687-3-7 This report is available online at: www.blood.gov.au For more information: Patient Blood Management National Blood Authority Locked Bag 8430 Canberra ACT 2601 Phone: 13000 BLOOD (13 000 25663) Email: [email protected] www.blood.gov.au Guidance for the provision of Intraoperative Cell Salvage Page 1 Ref No: Enter Organisation Ref AUS ICS Version No: 1 March 2014 Enter Organisation details here Guidance for the provision of Intraoperative Cell Salvage Author: Policy ratified by: Responsible Officer: Signature Date Insert Date Classification Clinical Date Issued Area Applicable Review Date Ref No: Version No: Disclaimer When using this document please ensure that the version you are using is the current, in date version by checking on your Organisation’s database for any new versions. -

Important Information for Female Platelet Donors
Important Information for Female Platelet Donors We are grateful for the support you provide our community blood program and especially appreciate your willingness to help save lives as a volunteer platelet donor. We also take our responsibility to provide a safe and adequate blood supply very seriously and need to share the following information regarding a change to our donor eligibility criteria for female platelet donors. We recently began performing a Human Leukocyte Antigen (HLA) antibody test on each of our current female platelet donors who have ever been pregnant. In addition, we modified our Medical History Questionnaire to ask donors whether they have been pregnant since their last donation. Platelet donors who respond yes to that question will be screened for HLA antibodies. Platelet donors will also be retested after every subsequent pregnancy. These adjustments are being made as part of our effort to reduce occurrences of Transfusion-Related Acute Lung Injury (TRALI). TRALI is a rare but serious complication of blood transfusions most commonly thought to be caused by a reaction to HLA antibodies present in the donor’s plasma. When transfused, these antibodies can sometimes cause plasma to leak into the patient’s lungs, creating fluid accumulation — a condition referred to as acute pulmonary edema. Female donors who have been pregnant are more likely than others to have these HLA antibodies in their plasma. Once the antibodies develop, they are present forever. The antibodies could be harmful if transfused into certain patients. The antibodies are present in plasma — and platelet donations contain a high volume of plasma, so our current efforts are directed at screening blood samples from female platelet donors to test for the HLA antibody. -

Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/Jp-Journals-10071-23250
INVITED ARTICLE Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/jp-journals-10071-23250 INTRODUCTION Department of Intensive Care Unit, Manipal Hospital, Bengaluru, Transfusion of whole blood is not associated with any significant Karnataka, India benefit, rather can be harmful. Significant advances have been Corresponding Author: Sunil Karanth, Department of Intensive made in transfusion medicine, facilitating the use of blood product Care Unit, Manipal Hospital, Bengaluru, Karnataka, India, e-mail: or component therapy than use of whole blood (Fig. 1). In 2009, a [email protected] report on Serious Hazards of Transfusion in the UK, estimated that How to cite this article: Karanth S. Transfusion Triggers for Platelets a total of 3 million units of blood components were released. The and Other Blood Products. Indian J Crit Care Med 2019;23(Suppl requirement of the same in South-East Asia is much higher to the 3):S189–S190. tune of 15 million units annually. The current recommendation Source of support: Nil is to use blood only in life-threatening situations, rather than to Conflict of interest: None normalize abnormal numbers. PLATELETS Platelets are derived from the buffy coat of whole blood donations. A pooled platelet concentrate includes pooled buffy-coat derived platelets from four whole-blood donations suspended in platelet additive solution and plasma of one of the four donors. It contains 240000 platelets pooled from 4–6 donors. A Single donor platelet is derived from a single-donor by a process of apheresis. In view of the lesser number of donors and the theoretical advantage of involving a single-donor platelet (SDP) may be preferred over the use of platelets from multiple donors. -

Blood-Platelet-Orders-Outpatient-V3
Blood / Platelet Orders Outpt v3 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED IN THE INFUSION CENTER PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending ___________________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Aplastic anemia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Medications diphenhydrAMINE HCl (Benadryl) 25 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 50 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 25 mg intravenously single dose premedicate prior to transfusion diphenhydrAMINE -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses.