Platelet Transfusion in the Real Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Transfusions
Guidelines for Transfu- sion Prepared by: Community Transfusion Committee Lincoln, Nebraska CHAIR: Aina Silenieks, MD MEMBERS: L. Bausch, M.D. R. Burton, M.D. S. Dunder, M.D. D. Voigt, M.D. B. J. Wilson, M.D. COMMUNITY Becky Croner REPRESENTATIVES: Ellen DiSalvo Christa Engel Phyllis Ericson Kelly Gillaspie Pat Gilles Vic Grdina Jessica Henrichs Kelly Jensen Laurel McReynolds Christina Nickel Angela Novotny Kelley Thiemann Janet Wachter Jodi Wikoff Guidelines For Transfusion Community Transfusion Committee INTRODUCTION The Community Transfusion Committee is a multidisciplinary group that meets to monitor blood utilization practices, establish guidelines for transfusion and discuss relevant transfusion related topics. It is comprised of physicians from local hospitals, invited guests, and community representatives from the hospitals’ transfusion services, nursing services, perfusion services, health information management, and the Nebraska Community Blood Bank. These Guidelines for Transfusion are reviewed and revised biannually by the Community Trans- fusion Committee to ensure that the industry’s most current practices are promoted. The Guidelines are the standard by which utilization practices are evaluated. They are also de- signed to provide helpful information to assist physicians to provide appropriate blood compo- nent therapy to patients. Appendices have been added for informational purposes and are not to be used as guidance for clinical decision making. ADULT RED CELLS A. Indications 1. One of the following a. Hypovolemia and hypoxia (signs/symptoms: syncope, dyspnea, postural hypoten- sion, tachycardia, angina, or TIA) secondary to surgery, trauma, GI tract bleeding, or intravascular hemolysis, OR b. Evidence of acute loss of 15% of total blood volume or >750 mL blood loss, OR c. -

Platelet Transfusion
Lab Dept: Transfusion Services Test Name: PLATELET TRANSFUSION General Information Lab Order Codes: TPLT Special charges: HLA Matched-MHLA, AHLA PLA1 Negative – PLAT, APLA1 Platelet Crossmatching PLTX – CPLT Synonyms: Leukocyte Reduced Platelets; Random Platelets; Platelet Rich Plasma; Platelet concentrate; Leukocyte Reduced Platelet Pheresis; Single- Donor Platelets; SDP Platelet pheresis; Apheresis Platelets; LRPH CPT Codes: P9035 – Platelet pheresis, Leukocyte reduced 86806 – Lymphocytotoxicity assay, visual crossmatch, without titration 86813 – HLA Matched 86945 – Irradiation 86985 – Volume Reduction 86965 – Pooling 86903 – Platelet Antigen typing 86999 – Washing 86022 – Platelet Crossmatch Test Includes: Leukocyte Reduced Platelet Pheresis consists of platelets suspended in 200-300 mL of plasma collected by cytapheresis. Each unit contains at least 3 x 1011 platelets, and ≤5.0 x 106 leukocytes. One pheresis unit equals 5-6 random unit platelet concentrate. Logistics Test Indications: Refer to Guidelines for the Transfusion of Blood Components. Platelet Crossmatching or HLA-Matched Platelets may be useful for patients receiving repeated platelet transfusions who have become refractile, and for patients who repeatedly develop febrile non-hemolytic transfusion reactions after platelet transfusions. Volume Reduced Platelets are indicated in the event that ABO - incompatible platelets must be transfused due to availability or in patients with severe fluid restrictions. Refer to Blood Component Compatibility Chart Lab Testing Sections: Transfusion Services Phone Numbers: MIN Lab: 612-813-6824 STP Lab: 651-220-6558 Test Availability: Daily, 24 hours Turnaround Time: 1 - 2 hours Standard Dose/Volume: <10 kg: 10 – 15 mL/kg up to one unit or 50 mL maximum 10 – 15 kg: 1/3 pheresis unit 15 – 25 kg: 1/2 pheresis unit >25 kg: 1 pheresis unit Rate of Infusion: 10 minutes/unit, <4 hours Administration: Must be administered through a blood component administration filter. -

Blood Banking/Transfusion Medicine Fellowship Program
DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE BLOOD BANKING/TRANSFUSION MEDICINE FELLOWSHIP PROGRAM THIS NEW ONE-YEAR ACGME-ACCREDITED FELLOWSHIP IN BLOOD BANKING/TRANSFUSION MEDICINE OFFERS STATE OF THE ART COMPREHENSIVE TRAINING IN BLOOD BANKING, COAGULATION, APHERESIS, AND HEMOTHERAPY AT THE MEMORIAL HERMANN HOSPITAL (MHH)-TEXAS MEDICAL CENTER (TMC) FOR PEDIATRICS AND ADULTS. This clinically-oriented fellowship is ideal for the candidate looking for exceptional experience in hemotherapy decision-making and coagulation consultation. We have a full spectrum of medical and surgical specialties, including a level 1 trauma center, as well as a busy solid organ transplant service (renal, liver, pancreas, cardiac, and lung). Fellows will rotate through: Our unique hemotherapy service: This innovative clinical consultative service for the Heart and Vascular Institute (HVI) allows fellows to serve as interventional blood banking consultants at the bed side as part of a multidisciplinary care team; our patients have complex bleeding and coagulopathy issues. Therapeutic apheresis service: This consultative service provide 24/7 direct patient care covering therapeutic plasmapheresis, red blood cell (RBC) exchanges, photopheresis, plateletpheresis, leukoreduction, therapeutic phlebotomies, and other related procedures on an inpatient and outpatient basis. We performed approximately over 1000 therapeutic plasma exchanges and RBC exchanges every year. Bloodbank: The MHH-TMC reference lab is one of the largest in Southeast Texas. This rotation provides extensive experience in interpreting antibody panel reports, working up transfusion reactions, investigating blood compatibility/incompatibility issues, and monitoring component usage. Gulf Coast Regional Blood Center: This rotation provides donor exposure in one of the largest community blood donation centers in the US as well as cellular therapy and immunohematology training. -

Transfusion Medicine/Blood Banking
Transfusion Medicine/Blood Banking REQUIRED rotation This is a onetime rotation and is not planned by PGY level. Objective: The objective of this rotation is to teach fellows the clinical and laboratory aspects of Transfusion Medicine and Blood Banking as it impacts hematology/oncology. Goals: The goal of this rotation is to teach fellows blood ordering practices, the difference of type and screen and type and cross matches and selection of appropriate products for transfusion. Fellows will also learn to perform, watch type and screen red cell antibodies and will learn the significance of ABO, Rh and other blood group antigen systems briefly so as to understand the significance of red cell antibodies in transfusion practices. Fellows will learn the different types of Blood components, their indications and contraindications and component modification. e.g. Irradiated and washed products, and special product request and transfusions e.g. granulocyte transfusion, CMV negative products etc. Fellows will learn and become proficient in the laboratory aspects of autoimmune hemolytic anemia, coagulopathies and bleeding disorders in relation to massive transfusions and the laboratory aspects and management of platelet refractoriness. Fellows will learn some aspects of coagulation factor replacement for factor deficiencies and inhibitors. Activities: Fellows will rotate in the Blood Bank at UMC and learn to perform, observe and interpret standard blood bank procedures such as ABO Rh typing, Antibody Screen, Antibody Identification and Direct Antiglobulin Test (DAT). Fellows will also watch platelet cross matching for platelet refractoriness. Fellows will attend and present a few didactic lectures of about 40 minutes on Blood Bank related topics as they apply to hematology/oncology like blood group antigens, Blood Component therapy, Transfusion reactions, Autoimmune hemolytic anemia, Platelet immunology and HLA as it applies to transfusion medicine. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Current Practice in Transfusion Medicine April 2019
CURRENT PRACTICE IN TRANSFUSION MEDICINE APRIL 2019 Faculty/Speakers Luzmary Alverez Suzanne A. Arinsburg, DO Assistant Director, Blood Bank and Transfusion Services - Mount Sinai Hospital; Assistant Professor of Pathology - Icahn School of Medicine at Mount Sinai Scott Avecilla, MD, PhD Medical Director of Cellular Therapy and Assistant Medical Director of Transfusion Medicine, Department of Laboratory Medicine - Memorial Sloan Kettering Cancer Center Ian Bain, MD, PhD Ian received his medical training and PhD at the Albert Einstein College of Medicine in the Bronx, with his research work focusing on the transcriptional mechanisms underlying anergy in natural Regulatory T cells. He completed his training in Clinical Pathology, as well as subspecialty training in Molecular Genetic Pathology. Currently is a fellow in Transfusion Medicine and Apheresis at Yale-New Haven. He will be transitioning to a role as an Assistant Professor in the Department of Pathology at Mt Sinai Hospital/Icahn School of Medicine with a focus on transfusion medicine, apheresis and cell therapy. His academic interests center around mechanisms and sources of red cell alloimmunization, as well as the impact of novel immmunotherapies on autoantibody formation. Anna Burgos, MT(ASCP) SBB Senior Immunohematologist, Laboratory of Immunohematology and Genomics – New York Blood Center Carmelita Carrier, PhD Director, Fred H. Allen Laboratory of Immunogenetics, New York Blood Center Connie Cai Immunohematology, New York Blood Center Stephanie Dormesy Manager-Apheresis & -

Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/Jp-Journals-10071-23250
INVITED ARTICLE Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/jp-journals-10071-23250 INTRODUCTION Department of Intensive Care Unit, Manipal Hospital, Bengaluru, Transfusion of whole blood is not associated with any significant Karnataka, India benefit, rather can be harmful. Significant advances have been Corresponding Author: Sunil Karanth, Department of Intensive made in transfusion medicine, facilitating the use of blood product Care Unit, Manipal Hospital, Bengaluru, Karnataka, India, e-mail: or component therapy than use of whole blood (Fig. 1). In 2009, a [email protected] report on Serious Hazards of Transfusion in the UK, estimated that How to cite this article: Karanth S. Transfusion Triggers for Platelets a total of 3 million units of blood components were released. The and Other Blood Products. Indian J Crit Care Med 2019;23(Suppl requirement of the same in South-East Asia is much higher to the 3):S189–S190. tune of 15 million units annually. The current recommendation Source of support: Nil is to use blood only in life-threatening situations, rather than to Conflict of interest: None normalize abnormal numbers. PLATELETS Platelets are derived from the buffy coat of whole blood donations. A pooled platelet concentrate includes pooled buffy-coat derived platelets from four whole-blood donations suspended in platelet additive solution and plasma of one of the four donors. It contains 240000 platelets pooled from 4–6 donors. A Single donor platelet is derived from a single-donor by a process of apheresis. In view of the lesser number of donors and the theoretical advantage of involving a single-donor platelet (SDP) may be preferred over the use of platelets from multiple donors. -

Blood-Platelet-Orders-Outpatient-V3
Blood / Platelet Orders Outpt v3 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED IN THE INFUSION CENTER PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending ___________________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Aplastic anemia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Medications diphenhydrAMINE HCl (Benadryl) 25 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 50 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 25 mg intravenously single dose premedicate prior to transfusion diphenhydrAMINE -
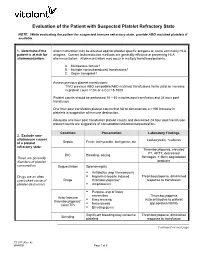
Platelet Transfusion Refractoriness
Evaluation of the Patient with Suspected Platelet Refractory State NOTE: While evaluating the patient for suspected immune refractory state, provide ABO matched platelets if available. 1. Determine if the Alloimmunization may be directed against platelet specific antigens or, more commonly HLA patient is at risk for antigens. Current leukoreduction methods are generally effective at preventing HLA alloimmunization: alloimmunization. Alloimmunization may occur in multiply transfused patients. A. Multiparous female? B. Multiple nonleukoreduced transfusions? C. Organ transplant? Assess previous platelet transfusions: TWO previous ABO compatible/ABO matched transfusions fail to yield an increase in platelet count >10K or a CCI >5-7500 Platelet counts should be performed 10 – 60 minutes post transfusion and 24 hour post transfusion. One hour post transfusion platelet counts that fail to demonstrate a >10K increase in platelets is suggestive of immune destruction. Adequate one hour post transfusion platelet counts and decreased 24 hour post transfusion platelet counts are suggestive of consumption/utilization/sequestration. Condition Presentation Laboratory Findings 2. Exclude non- alloimmune causes Leukocytosis, +cultures Sepsis Fever, tachycardia, tachypnea, etc. of a platelet refractory state: Thrombocytopenia, elevated PT, APTT, decreased DIC Bleeding, oozing These are generally fibrinogen, + fibrin degradation disorders of platelet products consumption. Sequestration Splenomegaly . Antibiotics (esp Vancomycin) Drugs are an often . Heparin (Heparin induced Thrombocytopenia, diminished overlooked cause of Drugs thrombocytopenia)* response to transfusion platelet destruction. Amphotericin . Purpura, esp of lower extremities Thrombocytopenia Auto-Immune . Easy bruising Auto antibodies to platelet thrombocytopenia* Nose bleeds glycoprotein IIb/IIIa (aka ITP) . Bleeding gums Significant bleeding may consume Thrombocytopenia, diminished Bleeding platelets response to transfusion Continued on next page TS 017 (Rev. -

Comparing Platelet Compatibility to Red Cell Compatibility Protocols
Review: comparing platelet compatibility to red cell compatibility protocols S. ROLIH Between 1971 and 1980, the number of platelet con- Incidence of Alloimmunization From centrates transfused in the United States increased from Transfusion 0.4 to 2.8 million units.1 That number increased to near- Each RBC or platelet transfusion has the potential to ly 5 million in 1987 and continues to increase in the influence the outcome of future transfusions of the 1990s. The increase in platelet transfusions has led to a same products by inducing the formation of alloanti- concomitant rise in the incidence of platelet alloimmu- bodies. For RBC transfusions, in which ABO and D nization. As a consequence, many transfusion services group-compatible units are routinely given, this is not a or blood product providers have developed platelet significant problem. Only 0.3 percent to 1.3 percent of compatibility protocols to select platelet components all RBC transfusion recipients produce alloantibodies, that will have acceptable survival when transfused to although in selected recipient populations, such as alloimmunized patients. Most serologists are familiar patients with thalassemia or sickle cell anemia, the with compatibility tests used to select red blood cells alloimmunization rate may be as high as 30 percent (RBCs) for transfusion. These tests are performed before because of chronic transfusions.2 Overall, decreased sur- transfusion, whether or not alloimmunization has vival of transfused RBCs due to alloimmunization occurs occurred. Platelet compatibility tests, in contrast, are infrequently. Once alloimmunization occurs, premature implemented only after alloimmunization and develop- loss of future transfused RBCs is prevented by the selec- ment of the refractory state. -

Outpatient Blood Platelet Orders
Blood / Platelet Orders Outpt v5 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED AS AN OUTPATIENT PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 ALL INFORMATION BELOW IS REQUIRED BY ORDERING PHYSICIAN Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending / Ordering Physician ______________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Myelodysplasia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Vital Signs Per BMH Policy, Blood and Blood Component Administration, 07.02 Diet Mediterranean Style Diet 2 Gm Sodium Diet Regular 7 Diet 1800 kcal Consistent Carbohydrate Diet Other Dietper patient choice ____________________ Medications -

Table 9: Transfusion-Associated Graft Versus Host Disease (Ta-Gvhd)
TABLE 9: TRANS FUS ION-AS S OCIATED GRAFT VERSUS HOST DISEASE (TA-GVHD) ALL PATIENTS SHOULD RECEIVE INFORMATION ON POTENTIAL TRANSFUSION REACTIONS AND HOW TO REPORT A SUSPECTED TRANSFUSION REACTION Signs & Symptoms Suggested Laboratory Possible Etiology Suggested Treatment & Actions Clinical Presentation Investigations . Fever TA-GVHD results from the infusion . Consult Physician . Monitor HGB, platelets & white cell Gastrointestinal tract: of viable T lymphocytes within . Detailed transfusion history count . Anorexia cellular blood components (RBC . Implement therapeutic interventions as . Monitor LFT . Nausea/Vomiting and platelets) into patients who are ordered by physician . Abdominal pain highly immunosuppressed or cannot . Continue to monitor patient for: . Diagnosis can be made by biopsy of . Profuse watery diarrhea which may be recognize the donor lymphocytes as . emerging S&S skin, liver, or bone marrow bloody foreign and reject them, allowing the . deterioration in patient’s condition Skin: donor lymphocytes to engraft in the . Definitive diagnosis of TA-GVHD . response to interventions . Erythematous, maculopapular rash patient. TA-GVHD occurs when the requires identification of donor derived that starts on the trunk & extends to patient HLA antigens are . 1 EDTA tube to Hematology for Complete lymphocytes in the patient peripheral the extremities, palms of hands & soles recognized as foreign by the blood count blood or tissues (by HLA typing) of feet engrafted donor lymphocytes, which . 1 (green) tube to Chemistry for LFT’s Liver: then attack the patient’s cells. Complete Transfusion Reaction Report Form** . Elevated liver function tests . Document event in patient records . Hepatocellular damage Immunocompetent patients may . Suggest referral to hematologist Bone marrow: develop TA-GVHD if they have .