Primer of Blood Administration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Requirements for Blood and Blood Components Intended for Transfusion Or for Further Manufacturing Use
Requirements for Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use Office of Blood Research and Review CBER, FDA Ask the FDA Session – AABB Annual Meeting October 26, 2015 1 Outline • Overview – Historical Background – Intent of the Rule – Organization of the Rule • Selected Provisions – Relevant Transfusion-Transmitted Infection – Control of Bacterial Contamination of Platelets – Medical Supervision – Donor Acknowledgement – Alternative Procedures – Donor Eligibility • Implementation 2 GAO Oversight and FDA Concerns • Publish in the form of regulations the guidelines that FDA deems essential to ensure the safety of the blood supply • Concern about the delay in requiring testing for emerging infectious agents, e.g. HTLV • Concern about blood safety and the regulations being out-of-date • Concerns about donor safety 3 Intent of the Final Rule • To better assure the safety of the blood supply and to help protect donor health • To make donor eligibility and testing requirements more consistent with current practices and to provide flexibility with regard to emerging infectious diseases • To accommodate technological advances • To establish requirements for donor education, donor history, and donor testing 4 Organization of the Final Rule - 1 • A. General • B. Definitions (§§ 606.3, 610.39, 630.3, 640.125) • C. Standard Operating Procedures (§ 606.100) • D. Control of Bacterial Contamination of Platelets (§ 606.145) • E. Records (§ 606.160) • F. Test Requirements (§§ 610.40, 640.5, 640.71(a)) • G. Donor Deferral (§ 610.41) 5 Organization of the Final Rule - 2 • H. Purpose and Scope (§ 630.1) • I. Medical Supervision (§ 630.5) • J. General Donor Eligibility Requirements(§ 630.10) • K. Donor Eligibility Requirements Specific to Whole Blood, Red Blood Cells and Plasma Collected by Apheresis (§ 630.15) • L. -

27. Clinical Indications for Cryoprecipitate And
27. CLINICAL INDICATIONS FOR CRYOPRECIPITATE AND FIBRINOGEN CONCENTRATE Cryoprecipitate is indicated in the treatment of fibrinogen deficiency or dysfibrinogenaemia.1 Fibrinogen concentrate is licenced for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenaemia and hypofibrinogenaemia,2 and is currently funded under the National Blood Agreement. Key messages y Fibrinogen is an essential component of the coagulation system, due to its role in initial platelet aggregation and formation of a stable fibrin clot.3 y The decision to transfuse cryoprecipitate or fibrinogen concentrate to an individual patient should take into account the relative risks and benefits.3 y The routine use of cryoprecipitate or fibrinogen concentrate is not advised in medical or critically ill patients.2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In the setting of major obstetric haemorrhage, early administration of cryoprecipitate or fibrinogen concentrate may be necessary.3 Clinical implications y The routine use of cryoprecipitate or fibrinogen concentrate in medical or critically ill patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of disseminated intravascular coagulopathy (MED-PP18, CC-PP7).2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In patients with critical bleeding requiring massive transfusion, suggested doses of blood components is 3-4g (CBMT-PP10)3 in adults or as per the local Massive Transfusion Protocol. -
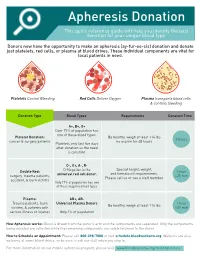
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Changes to the Technical Manual, 18Th Edition Monday, November 17, 2014 12:00 P.M
Changes to the Technical Manual, 18th Edition Monday, November 17, 2014 12:00 p.m. – 1:30 p.m. (ET) / 5:00p.m. – 6:30 p.m. (GMT) When this file is opened, Acrobat Reader will, by default, display the slides including the Acrobat reader controls. To return to full screen mode, hit Ctrl-L on your computer keyboard or use your mouse to click View>Full Screen on the menu bar of the Acrobat Reader program. To take the slides out of full screen mode and display the Acrobat Reader controls, simply hit the Esc key on your computer keyboard. To advance slides during the program, use the Enter, Page Down, down arrow or right arrow on your computer keyboard. To back up slides during the program, use the Page Up, up arrow of left arrow on your computer keyboard. Please remember to logon to the Live Learning Center using your email address and password to complete an evaluation of the program and speakers. At this time, advance to the next slide and wait for the audioconference to begin. A 2014 Audioconference presented to you by AABB The AABB Technical Manual 18th Edition What’s new? www.aabb.org Technical Manual by the Numbers • 1953 =year of first edition • 69 = number of authors/editors this edition • 370,378 = word count • 96 = number of methods • 60 = number of countries where TM is used www.aabb.org It’s a Process www.aabb.org Overview of Changes Major • Molecular testing • Patient blood management • Cellular therapy • Methods Minor • Throughout www.aabb.org 1: Quality Systems 2: Facilities and Safety • These 2 chapters are comprehensive discussions -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Guidance for the Provision of Intraoperative Cell Salvage
Enter Organisation details here GUIDANCE FOR THE PROVISION OF INTRAOPERATIVE CELL SALVAGE Guidance Guidance forfor Australian Australian Health Health Providers Providers MARCH> MARCH 2014 2014 Guidance for the provision of Intraoperative Cell Salvage Page 0 Ref No: Enter Organisation Ref AUS ICS Version No: 1 March 2014 Enter Organisation details here With the exception of any logos and registered trademarks, and where otherwise noted, all material presented in this document is provided under a Creative Commons Attribution 3.0 Australia (http://creativecommons.org/ licenses/by/3.0/au/) licence. The details of the relevant licence conditions are available on the Creative Commons website (accessible using the links provided) as is the full legal code for the CC BY 3.0 AU license (http://creativecommons.org/licenses/by/3.0/au/legalcode). The content obtained from this document or derivative of this work must be attributed as the Guidance for the provision of Intraoperative Cell Salvage. © National Blood Authority, 2014. ISBN 978-0-9873687-3-7 This report is available online at: www.blood.gov.au For more information: Patient Blood Management National Blood Authority Locked Bag 8430 Canberra ACT 2601 Phone: 13000 BLOOD (13 000 25663) Email: [email protected] www.blood.gov.au Guidance for the provision of Intraoperative Cell Salvage Page 1 Ref No: Enter Organisation Ref AUS ICS Version No: 1 March 2014 Enter Organisation details here Guidance for the provision of Intraoperative Cell Salvage Author: Policy ratified by: Responsible Officer: Signature Date Insert Date Classification Clinical Date Issued Area Applicable Review Date Ref No: Version No: Disclaimer When using this document please ensure that the version you are using is the current, in date version by checking on your Organisation’s database for any new versions. -

Transfusion Guidelines Updated February, 2005
Transfusion Guidelines Updated February, 2005 I. Whole Blood The use of whole blood is not recommended and is not available from the Blood Center. Blood components should be selected according to the patient’s needs. II. Red Blood Cells (RBCs) Transfusion must be completed within 4 hours of issue from the Blood Bank. If transfusion is not begun immediately the RBCs must be stored at 1-6° C or returned to the Blood Bank. (Storage is only allowed in preapproved areas, such as the operating room and several of the critical care areas). The effectiveness of each transfusion should be documented in the medical record. Single unit transfusions of RBCs are often effective. In adult patients, one unit of RBCs will increase the hemoglobin level by approximately 1 g/dl (hematocrit by 3%). A. Acceptable Usage 1. Acute Blood loss exceeding 30-40% of blood volume (pediatric patients - 10-15 ml/Kg) and/or not responding to appropriate volume resuscitation, and/or with ongoing blood loss. 2. Patient is normovolemic and there is evidence to support a need for increased oxygen carrying capacity by the following : a. Hypotension not corrected by adequate volume replacement alone. b. PVO2<25 torr, when SaO2 completely saturated; extraction ratio>50%, VO2<50% of baseline. c. Neonates and young infants less than 56 weeks postmenstrual age with hematocrit < 0.30 and frequent and/or severe apnea/bradycardia, poor weight gain, sustained tachycardia and/or tachypnea or mild respiratory distress. d. Neonates and young infants less than 56 weeks postmenstrual age with hematocrit < 0.35 and moderate respiratory distress or with hematocrit < 0.40 and severe respiratory distress, cyanotic congenital heart disease or receiving extracorporeal membrane oxygenation. -
Laboratory Best Transfusion Practice for Neonates, Infants and Children
Laboratory Best Transfusion Practice for Neonates, Infants and Children This summary guidance should be used in conjunction with the appropriate 20161 and 20122 BSH Guidelines and laboratory SOPs Compatibility testing Neonates and infants < 4 months Obtain neonatal and maternal transfusion history (including any fetal transfusions) for all admissions. Obtain a maternal sample for initial testing where possible, in addition to the patient sample. Red cell selection: no maternal antibodies present Select appropriate group and correct neonatal specification red cells. Group O D-negative red cells may be issued electronically without serological crossmatch. If the laboratory does not universally select group O D-negative red cells for this age group, blood group selection should either be controlled by the LIMS or an IAT crossmatch should be performed using maternal or neonatal plasma to serologically confirm ABO compatibility with both mother and neonate. Red cell selection: where there is maternal antibody Select appropriate group red cells, compatible with maternal alloantibody/ies. An IAT crossmatch should be performed using the maternal plasma. If it is not possible to obtain a maternal sample it is acceptable to crossmatch antigen-negative units against the infant’s plasma. Where paedipacks are being issued from one donor unit it is only necessary to crossmatch the first split pack. Subsequent split packs from this multi-satellite unit can be automatically issued without further crossmatch until the unit expires or the infant is older than 4 months. If packs from a different donor are required, an IAT crossmatch should be performed. Infants and children ≥ 4 months For infants and children from 4 months of age, pre-transfusion testing and compatibility procedures should be performed as recommended for adults. -

Cryoprecipitate
VUMC Blood Bank Website Products Page Cryoprecipitate Dosage: The VUMC blood bank maintains two distinct cryoprecipitate products. Adult patients will receive pre-pooled units of cryoprecipitate, these units contain 5 individual cryo units. Providers caring for adult patients can order pre-pooled cryoprecipitate in increments, with a traditional order of 2 pre-pooled cryoprecipitate units for an adult patient. Pediatric patients at VUMC receive cryoprecipitate via individual units according to weight based transfusion guidelines (recommended 10-15 mL/kg). Orders for cryoprecipitate that deviate from this algorithm - as well as orders for cryoprecipitate for patients without a recent fibrinogen level document in Starpanel - are flagged for review by the blood bank resident and/or the medical director. Introduction: According to standards set by the AABB, each unit of cryoprecipitate must contain at least 150 mg of fibrinogen. Cryoprecipitate also contains at least 80 IU of Factor VIII and appreciable amounts of von Willebrand Factor (vWF) and Factor XIII. Cryoprecipitate does not contain appreciable amounts of the other clotting factors. Indications: The most common indication for cryoprecipitate transfusion is hypofibrinogenemia, usually in the setting of DIC or major surgery but occasionally do to hereditary hypofibrinogenemia. Less commonly, cryoprecipitate has been used to provide factor replacement in Factor XIII deficiency. Please note, that human factor XIII concentrates are FDA approved for maintenance therapy (www.corifact.com). Cryoprecipitate should NOT be used for treatment of hemophilia A (Factor VIII deficiency) or von Willebrand’s disease. Vanderbilt discourages the use of cryoprecipitate as a post-surgical fibrin sealant. Special Information Unlike RBCs, platelets, and FFP, once cryoprecipitate is thawed, it cannot be re-stocked (re-frozen) by the blood bank. -

Cord Blood Stem Cell Transplantation
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights • Umbilical cord blood, like bone marrow and peripheral blood, is a rich source of stem cells for transplantation. There may be advantages for certain patients to have cord blood stem cell transplants instead of transplants with marrow or peripheral blood stem cells (PBSCs). • Stem cell transplants (peripheral blood, marrow or cord blood) may use the patient’s own stem cells (called “autologous transplants”) or use donor stem cells. Donor cells may come from either a related or unrelated matched donor (called an “allogeneic transplant”). Most transplant physicians would not want to use a baby’s own cord blood (“autologous transplant”) to treat his or her leukemia. This is because donor stem cells might better fight the leukemia than the child’s own stem cells. • Cord blood for transplantation is collected from the umbilical cord and placenta after a baby is delivered. Donated cord blood that meets requirements is frozen and stored at a cord blood bank for future use. • The American Academy of Pediatrics’s (AAP) policy statement (Pediatrics; 2007;119:165-170.) addresses public and private banking options available to parents. Among several recommendations, the report encourages parents to donate to public cord blood banks and discourages parents from using private cord blood banks for personal or family cord blood storage unless they have an older child with a condition that could benefit from transplantation. • The Stem Cell Therapeutic and Research Act of 2005 put several programs in place, including creation of the National Cord Blood Inventory (NCBI) for patients in need of transplantation. -

Therapeutic Apheresis, J Clin Apheresis 2007, 22, 104-105
Apheresis: Basic Principles, Practical Considerations and Clinical Applications Joseph Schwartz, MD Anand Padmanabhan, MD PhD Director, Transfusion Medicine Assoc Med Director/Asst Prof Columbia Univ. Medical Center BloodCenter of Wisconsin New York Presbyterian Hospital Medical College of Wisconsin Review Session, ASFA Annual meeting, Scottsdale, Arizona, June 2011 Objectives (Part 1) • Mechanism of Action • Definitions • Technology (ies) • Use • Practical Considerations • Math • Clinical applications – HPC Collection Objectives (Part 2) • Clinical applications: System/ Disease Specific Indications • ASFA Fact Sheet Apheresis •Derives from Greek, “to carry away” •A technique in which whole blood is taken and separated extracorporealy, separating the portion desired from the remaining blood. •This allows the desired portion (e.g., plasma) to be removed and the reminder returned. Apheresis- Mechanism of Action •Large-bore intravenous catheter connected to a spinning centrifuge bowl •Whole blood is drawn from donor/patient into the centrifuge bowl •The more dense elements, namely the RBC, settle to the bottom with less dense elements such as WBC and platelets overlying the RBC layer and finally, plasma at the very top. Apheresis: Principles of Separation Platelets (1040) Lymphocytes Torloni MD (1050-1061) Monocytes (1065 - 1069) Granulocyte (1087 - 1092) RBC Torloni MD Torloni MD Separate blood components is based on density with removal of the desired component Graphics owned by and courtesy of Gambro BCT Principals of Apheresis WBC Plasma Torlo RBC ni MD Torloni MD RBC WBC Plasma G Cobe Spectra Apheresis- Mechanism of Action Definitions • Plasmapheresis: plasma is separated, removed (i.e. less than 15% of total plasma volume) without the use of replacement solution • Plasma exchange (TPE): plasma is separated, removed and replaced with a replacement solution such as colloid (e.g. -

View Table of Contents
Table of Contents Preface. xv About the Authors . xvii Section 1: ABO: The Birth of Blood Groups 1. Blood Groups—From Then ’Til Now . .1 Discovery . .1 Response and Reconsideration . .2 Beyond ABO . .4 M, N, and P . .5 Rh . .6 The Antiglobulin Test . .7 The Plot Thickens . .7 References . .8 2. Karl Landsteiner, Father of Blood Groups . .9 Beginnings . .9 Medical School . .11 The Young Researcher . .13 Priority Issues . .16 Decastello and Sturli: Another Blood Group . .18 Other Directions . .19 Life in Vienna . .21 References . .23 3. ABO Grows Up . .27 Transfusion History, 1600 to 1920 . .27 The Blood Groups Gain Attention . .29 Transfusion in World War I . .37 Advances in ABO Blood Group Serology . .39 References . .47 4. Genetics, Eugenics, and Blood Groups . .51 Heredity, 1900 . .52 The Rise of Genetics . .52 Genes and Blood Groups . .53 viii BLOODY BRILLIANT! Genes and Populations . 59 References . 62 5. Karl Landsteiner in New York . 65 At “The Rockefeller” . 65 Landsteiner Returns to the Blood Groups . 68 The Nobel Prize . 71 The Laureate at Work . 73 References . 74 6. Out, Damned Spot! Early Forensic Applications of ABO . 77 What Blood Can Tell . 78 Is It Blood? . 78 Is It Human Blood? . 79 Whose Blood Is It? . 81 References . 85 7. Who’s Your Daddy? ABO and Paternity Testing . 87 Establishing Paternity . 87 Paternity Testing and the Courts: Europe . 89 Paternity Testing and the Courts: Britain . 91 Paternity Testing and the Courts: United States . 92 Lights! Camera! Action! . ..