Observational Studies and How Do They Differ from Clinical Trials?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
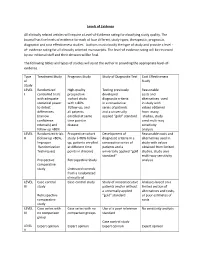
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Top Ten Cool Things About 250B Exam 1
Cohort Studies Madhukar Pai, MD, PhD McGill University Montreal 1 Cohort: origins of the word http://www.caerleon.net/ 2 Introduction • Measurement of the occurrence of events over time is a central goal of epidemiologic research • Regardless of any particular study design or hypothesis, interest is ultimately in the disease or outcome-causing properties of factors that are antecedent to the disease or outcome. • All study designs (including case control and cross- sectional studies) are played out in some populations over time (either well defined cohorts or not) – They differ in how they acknowledge time and how they sample exposed and non-exposed as these groups develop disease over time 3 All the action happens within a “sea of person-time” in which events occur 4 Morgenstern IJE 1980 Cohort studies Intuitive approach to studying disease incidence and risk factors: 1. Start with a population at risk 2. Measure exposures and covariates at baseline 3. Follow-up the cohort over time with a) Surveillance for events or b) re-examination 4. Keep track of attrition, withdrawals, drop-outs and competing risks 5. For covariates that change over time, measure them again during follow up 6. Compare event rates in people with and without exposures of interest - Incidence Density Ratio (IDR) is the most natural and appropriate measure of effect - Adjust for confounders and compute adjusted IDR - Look for effect measure modification, if appropriate 5 Cohort studies • Can be large or small • Can be long or short duration • Can be simple or elaborate • Can look at multiple exposures and multiple outcomes • Can look at changes in exposures over time • For rare outcomes need many people and/or lengthy follow- up • Are usually very expensive because of the numbers and follow-up requirements • But once a cohort is established, can sustain research productivity for a long, long time! 6 Cohort: keeping track of people 7 Szklo & Nieto. -

Observational Studies and Bias in Epidemiology
The Young Epidemiology Scholars Program (YES) is supported by The Robert Wood Johnson Foundation and administered by the College Board. Observational Studies and Bias in Epidemiology Manuel Bayona Department of Epidemiology School of Public Health University of North Texas Fort Worth, Texas and Chris Olsen Mathematics Department George Washington High School Cedar Rapids, Iowa Observational Studies and Bias in Epidemiology Contents Lesson Plan . 3 The Logic of Inference in Science . 8 The Logic of Observational Studies and the Problem of Bias . 15 Characteristics of the Relative Risk When Random Sampling . and Not . 19 Types of Bias . 20 Selection Bias . 21 Information Bias . 23 Conclusion . 24 Take-Home, Open-Book Quiz (Student Version) . 25 Take-Home, Open-Book Quiz (Teacher’s Answer Key) . 27 In-Class Exercise (Student Version) . 30 In-Class Exercise (Teacher’s Answer Key) . 32 Bias in Epidemiologic Research (Examination) (Student Version) . 33 Bias in Epidemiologic Research (Examination with Answers) (Teacher’s Answer Key) . 35 Copyright © 2004 by College Entrance Examination Board. All rights reserved. College Board, SAT and the acorn logo are registered trademarks of the College Entrance Examination Board. Other products and services may be trademarks of their respective owners. Visit College Board on the Web: www.collegeboard.com. Copyright © 2004. All rights reserved. 2 Observational Studies and Bias in Epidemiology Lesson Plan TITLE: Observational Studies and Bias in Epidemiology SUBJECT AREA: Biology, mathematics, statistics, environmental and health sciences GOAL: To identify and appreciate the effects of bias in epidemiologic research OBJECTIVES: 1. Introduce students to the principles and methods for interpreting the results of epidemio- logic research and bias 2. -

Study Types Transcript
Study Types in Epidemiology Transcript Study Types in Epidemiology Welcome to “Study Types in Epidemiology.” My name is John Kobayashi. I’m on the Clinical Faculty at the Northwest Center for Public Health Practice, at the School of Public Health and Community Medicine, University of Washington in Seattle. From 1982 to 2001, I was the state epidemiologist for Communicable Diseases at the Washington State Department of Health. Since 2001, I’ve also been the foreign adviser for the Field Epidemiology Training Program of Japan. About this Module The modules in the epidemiology series from the Northwest Center for Public Health Practice are intended for people working in the field of public health who are not epidemiologists, but who would like to increase their understanding of the epidemiologic approach to health and disease. This module focuses on descriptive and analytic epide- miology and their respective study designs. Before you go on with this module, we recommend that you become familiar with the following modules, which you can find on the Center’s Web site: What is Epidemiology in Public Health? and Data Interpretation for Public Health Professionals. We introduce a number of new terms in this module. If you want to review their definitions, the glossary in the attachments link at the top of the screen may be useful. Objectives By now, you should be familiar with the overall approach of epidemiology, the use of various kinds of rates to measure disease frequency, and the various ways in which epidemiologic data can be presented. This module offers an overview of descriptive and analytic epidemiology and the types of studies used to review and investigate disease occurrence and causes. -

Chapter 5 Experiments, Good And
Chapter 5 Experiments, Good and Bad Point of both observational studies and designed experiments is to identify variable or set of variables, called explanatory variables, which are thought to predict outcome or response variable. Confounding between explanatory variables occurs when two or more explanatory variables are not separated and so it is not clear how much each explanatory variable contributes in prediction of response variable. Lurking variable is explanatory variable not considered in study but confounded with one or more explanatory variables in study. Confounding with lurking variables effectively reduced in randomized comparative experiments where subjects are assigned to treatments at random. Confounding with a (only one at a time) lurking variable reduced in observational studies by controlling for it by comparing matched groups. Consequently, experiments much more effec- tive than observed studies at detecting which explanatory variables cause differences in response. In both cases, statistically significant observed differences in average responses implies differences are \real", did not occur by chance alone. Exercise 5.1 (Experiments, Good and Bad) 1. Randomized comparative experiment: effect of temperature on mice rate of oxy- gen consumption. For example, mice rate of oxygen consumption 10.3 mL/sec when subjected to 10o F. temperature (Fo) 0 10 20 30 ROC (mL/sec) 9.7 10.3 11.2 14.0 (a) Explanatory variable considered in study is (choose one) i. temperature ii. rate of oxygen consumption iii. mice iv. mouse weight 25 26 Chapter 5. Experiments, Good and Bad (ATTENDANCE 3) (b) Response is (choose one) i. temperature ii. rate of oxygen consumption iii. -

Analytic Epidemiology Analytic Studies: Observational Study
Analytic Studies: Observational Study Designs Cohort Studies Analytic Epidemiology Prospective Cohort Studies Retrospective (historical) Cohort Studies Part 2 Case-Control Studies Nested case-control Case-cohort studies Dr. H. Stockwell Case-crossover studies Determinants of disease: Analytic Epidemiology Epidemiology: Risk factors Identifying the causes of disease A behavior, environmental exposure, or inherent human characteristic that is associated with an important health Testing hypotheses using epidemiologic related condition* studies Risk factors are associated with an increased probability of disease but may Goal is to prevent disease (deterrents) not always cause the diseases *Last, J. Dictionary of Epidemiology Analytic Studies: Cohort Studies Panel Studies Healthy subjects are defined by their Combination of cross-sectional and exposure status and followed over time to cohort determine the incidence of disease, symptoms or death Same individuals surveyed at several poiiiints in time Subjects grouped by exposure level – exposed and unexposed (f(reference group, Can measure changes in individuals comparison group) Analytic Studies: Case-Control Studies Nested case-control studies A group of individuals with a disease Case-control study conducted within a (cases) are compared with a group of cohort study individuals without the disease (controls) Advantages of both study designs Also called a retrospective study because it starts with people with disease and looks backward for ppprevious exposures which might be -

Epidemiologic Study Designs
Epidemiologic Study Designs Jacky M Jennings, PhD, MPH Associate Professor Associate Director, General Pediatrics and Adolescent Medicine Director, Center for Child & Community Health Research (CCHR) Departments of Pediatrics & Epidemiology Johns Hopkins University Learning Objectives • Identify basic epidemiologic study designs and their frequent sequence of study • Recognize the basic components • Understand the advantages and disadvantages • Appropriately select a study design Research Question & Hypotheses Analytic Study Plan Design Basic Study Designs and their Hierarchy Clinical Observation Hypothesis Descriptive Study Case-Control Study Cohort Study Randomized Controlled Trial Systematic Review Causality Adapted from Gordis, 1996 MMWR Study Design in Epidemiology • Depends on: – The research question and hypotheses – Resources and time available for the study – Type of outcome of interest – Type of exposure of interest – Ethics Study Design in Epidemiology • Includes: – The research question and hypotheses – Measures and data quality – Time – Study population • Inclusion/exclusion criteria • Internal/external validity Epidemiologic Study Designs • Descriptive studies – Seeks to measure the frequency of disease and/or collect descriptive data on risk factors • Analytic studies – Tests a causal hypothesis about the etiology of disease • Experimental studies – Compares, for example, treatments EXPOSURE Cross-sectional OUTCOME EXPOSURE OUTCOME Case-Control EXPOSURE OUTCOME Cohort TIME Cross-sectional studies • Measure existing disease and current exposure levels at one point in time • Sample without knowledge of exposure or disease • Ex. Prevalence studies Cross-sectional studies • Advantages – Often early study design in a line of investigation – Good for hypothesis generation – Relatively easy, quick and inexpensive…depends on question – Examine multiple exposures or outcomes – Estimate prevalence of disease and exposures Cross-sectional studies • Disadvantages – Cannot infer causality – Prevalent vs. -

1 Sensitivity Analysis in Observational Research: Introducing the E-‐Value
Sensitivity Analysis in Observational Research: Introducing the E-value Tyler J. VanderWeele, Ph.D., Departments of Epidemiology and Biostatistics, Harvard T.H. Chan School of Public Health Peng Ding, Ph.D., Department of Statistics, University of California, Berkeley This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record: VanderWeele, T.J. and Ding, P. (2017). Sensitivity analysis in observational research: introducing the E-value. Annals of Internal Medicine, 167(4):268-274. 1 Abstract. Sensitivity analysis can be useful in assessing how robust associations are to potential unmeasured or uncontrolled confounding. In this paper we introduce a new measure that we call the “E-value,” a measure related to the evidence for causality in observational studies, when they are potentially subject to confounding. The E-value is defined as the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the treatment and the outcome to fully eXplain away a specific treatment-outcome association, conditional on the measured covariates. -

Chapter 20 Design and Analysis of Experiments and Observational
Chapter 20 Design and Analysis of Experiments and Observational Studies Point of both observational studies and designed experiments is to identify variable or set of variables, called explanatory variables, which are thought to predict outcome or response variable. Observational studies are an investigation of the association between treatment and response, where subject who decide whether or not they take a treatment. In experimental designs, experimenter decides who is given a treat- ment and who is to be a control, the experimenter alters levels of treatments when investigating how these alterations cause change in the response. 20.1 Observational Studies Exercise 20.1 (Observational Studies) 1. Observational Study versus Designed Experiment. (a) Effect of air temperature on rate of oxygen consumption (ROC) of four mice is investigated. ROC of one mouse at 0o F is 9.7 mL/sec for example. temperature (Fo) 0 10 20 30 ROC (mL/sec) 9.7 10.3 11.2 14.0 Since experimenter (not a mouse!) decides which mice are subjected to which temperature, this is (choose one) observational study / designed experiment. (b) Indiana police records from 1999–2001 on six drivers are analyzed to de- termine if there is an association between drinking and traffic accidents. One heavy drinker had 6 accidents for example. 169 170Chapter 20. Design and Analysis of Experiments and Observational Studies (lecture notes 10) drinking → heavy light 3 1 6 2 2 1 This is an observational study because (choose one) i. police decided who was going to drink and drive and who was not. ii. drivers decided who was going to drink and drive and who was not. -

Overview of Epidemiological Study Designs
University of Massachusetts Medical School eScholarship@UMMS PEER Liberia Project UMass Medical School Collaborations in Liberia 2018-11 Overview of Epidemiological Study Designs Richard Ssekitoleko Yale University Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/liberia_peer Part of the Clinical Epidemiology Commons, Epidemiology Commons, Family Medicine Commons, Infectious Disease Commons, Medical Education Commons, and the Quantitative, Qualitative, Comparative, and Historical Methodologies Commons Repository Citation Ssekitoleko R. (2018). Overview of Epidemiological Study Designs. PEER Liberia Project. https://doi.org/ 10.13028/fp3z-mv12. Retrieved from https://escholarship.umassmed.edu/liberia_peer/5 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in PEER Liberia Project by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Overview of Epidemiological study Designs Richard Ssekitoleko Department of Global Health Yale University 1.1 Objectives • Understand the different epidemiological study types • Get to know what is involved in each type of study • Understand the strengths and Limitations of each study type Key terms • Population • Consists of all elements and is the group from which a sample is drawn • A sample is a subset of a population • Parameters • Summary data from a population • Statistics • Summary data from a sample • Validity • Extent to which a conclusion or statistic is well-founded and likely corresponds accurately to the parameter. Hierarchy of Evidence Study type Observational Interventional Descriptive Experiment Ecological Randomized Controlled Trial Cross-sectional Case-control Cohort Overview of Epidemiologic Study Designs Validity *anecdotes Cost Understanding What Physicians Mean: In my experience … once.