Cohort, Cross Sectional, and Case-Control Studies C J Mann
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
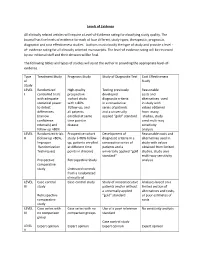
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

FDA Oncology Experience in Innovative Adaptive Trial Designs
Innovative Adaptive Trial Designs Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Office of Biostatistics, CDER, FDA 9/3/2015 Sridhara - Ovarian cancer workshop 1 Fixed Sample Designs • Patient population, disease assessments, treatment, sample size, hypothesis to be tested, primary outcome measure - all fixed • No change in the design features during the study Adaptive Designs • A study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (interim data) from subjects in the study 9/3/2015 Sridhara - Ovarian cancer workshop 2 Bayesian Designs • In the Bayesian paradigm, the parameter measuring treatment effect is regarded as a random variable • Bayesian inference is based on the posterior distribution (Bayes’ Rule – updated based on observed data) – Outcome adaptive • By definition adaptive design 9/3/2015 Sridhara - Ovarian cancer workshop 3 Adaptive Designs (Frequentist or Bayesian) • Allows for planned design modifications • Modifications based on data accrued in the trial up to the interim time • Unblinded or blinded interim results • Control probability of false positive rate for multiple options • Control operational bias • Assumes independent increments of information 9/3/2015 Sridhara - Ovarian cancer workshop 4 Enrichment Designs – Prognostic or Predictive • Untargeted or All comers design: – post-hoc enrichment, prospective-retrospective designs – Marker evaluation after randomization (example: KRAS in cetuximab -

TUTORIAL in BIOSTATISTICS: the Self-Controlled Case Series Method
STATISTICS IN MEDICINE Statist. Med. 2005; 0:1–31 Prepared using simauth.cls [Version: 2002/09/18 v1.11] TUTORIAL IN BIOSTATISTICS: The self-controlled case series method Heather J. Whitaker1, C. Paddy Farrington1, Bart Spiessens2 and Patrick Musonda1 1 Department of Statistics, The Open University, Milton Keynes, MK7 6AA, UK. 2 GlaxoSmithKline Biologicals, Rue de l’Institut 89, B-1330 Rixensart, Belgium. SUMMARY The self-controlled case series method was developed to investigate associations between acute outcomes and transient exposures, using only data on cases, that is, on individuals who have experienced the outcome of interest. Inference is within individuals, and hence fixed covariates effects are implicitly controlled for within a proportional incidence framework. We describe the origins, assumptions, limitations, and uses of the method. The rationale for the model and the derivation of the likelihood are explained in detail using a worked example on vaccine safety. Code for fitting the model in the statistical package STATA is described. Two further vaccine safety data sets are used to illustrate a range of modelling issues and extensions of the basic model. Some brief pointers on the design of case series studies are provided. The data sets, STATA code, and further implementation details in SAS, GENSTAT and GLIM are available from an associated website. key words: case series; conditional likelihood; control; epidemiology; modelling; proportional incidence Copyright c 2005 John Wiley & Sons, Ltd. 1. Introduction The self-controlled case series method, or case series method for short, provides an alternative to more established cohort or case-control methods for investigating the association between a time-varying exposure and an outcome event. -

FORMULAS from EPIDEMIOLOGY KEPT SIMPLE (3E) Chapter 3: Epidemiologic Measures
FORMULAS FROM EPIDEMIOLOGY KEPT SIMPLE (3e) Chapter 3: Epidemiologic Measures Basic epidemiologic measures used to quantify: • frequency of occurrence • the effect of an exposure • the potential impact of an intervention. Epidemiologic Measures Measures of disease Measures of Measures of potential frequency association impact (“Measures of Effect”) Incidence Prevalence Absolute measures of Relative measures of Attributable Fraction Attributable Fraction effect effect in exposed cases in the Population Incidence proportion Incidence rate Risk difference Risk Ratio (Cumulative (incidence density, (Incidence proportion (Incidence Incidence, Incidence hazard rate, person- difference) Proportion Ratio) Risk) time rate) Incidence odds Rate Difference Rate Ratio (Incidence density (Incidence density difference) ratio) Prevalence Odds Ratio Difference Macintosh HD:Users:buddygerstman:Dropbox:eks:formula_sheet.doc Page 1 of 7 3.1 Measures of Disease Frequency No. of onsets Incidence Proportion = No. at risk at beginning of follow-up • Also called risk, average risk, and cumulative incidence. • Can be measured in cohorts (closed populations) only. • Requires follow-up of individuals. No. of onsets Incidence Rate = ∑person-time • Also called incidence density and average hazard. • When disease is rare (incidence proportion < 5%), incidence rate ≈ incidence proportion. • In cohorts (closed populations), it is best to sum individual person-time longitudinally. It can also be estimated as Σperson-time ≈ (average population size) × (duration of follow-up). Actuarial adjustments may be needed when the disease outcome is not rare. • In an open populations, Σperson-time ≈ (average population size) × (duration of follow-up). Examples of incidence rates in open populations include: births Crude birth rate (per m) = × m mid-year population size deaths Crude mortality rate (per m) = × m mid-year population size deaths < 1 year of age Infant mortality rate (per m) = × m live births No. -

Supplementary File
Supplementary file Table S1. Characteristics of studies included in the systematic review. Authors, reference Type of article Number of patients Type of stroke Valderrama [1] case report 1 AIS Oxley [2] case series 5 AIS Morassi [3] case series 6 AIS (n = 4), HS (n = 2) Tunc [4] case series 4 AIS Avula [5] case series 4 AIS (n = 3), TIA (n = 1) Oliver [6] case report 1 AIS Beyrouti [7] case series 6 AIS Al Saiegh [8] case series 2 AIS (n = 1), SAH (n = 1) Gunasekaran [9] case report 1 AIS Goldberg [10] case report 1 AIS Viguier [11] case report 1 AIS Escalard [12] case series/ case control 10 AIS Wang [13] case series 5 AIS Fara [14] case series 3 AIS Moshayedi [15] case report 1 AIS Deliwala [16] case report 1 AIS Sharafi-Razavi [17] case report 1 HS retrospective cohort study (case Jain [18] 35 AIS (n = 26) HS (n = 9) control) retrospective cohort study (case Yaghi [19] 32 AIS control) AIS (n = 35), TIA (n = 5), HS Benussi [20] retrospective cohort study 43 (n = 3) Rudilosso [21] case report 1 AIS Lodigiani [22] retrospective cohort study 9 AIS Malentacchi [23] case report 1 AIS J. Clin. Med. 2020, 9, x; doi: FOR PEER REVIEW www.mdpi.com/journal/jcm J. Clin. Med. 2020, 9, x FOR PEER REVIEW 2 of 8 retrospective cohort study/case Scullen [24] 2 HS + AIS (n = 1), AIS (n = 1) series retrospective cohort study/ case AIS (n = 17), ST (n = 2), HS Sweid [25] 22 series (n = 3) AIS – acute ischemic stroke; HS – haemorrhagic stroke; SAH – subarachnoid haemorrhage; ST – sinus thrombosis; TIA-transient ischemic attack. -

The Very Process of Taking a Pill May Create Expectations in a Patient Which May Affect Reactions to the Pill
Ch 1 Pause for Thought Questions p. 36 1- Why is it important to use placebos and a double-blind approach in some studies? The very process of taking a pill may create expectations in a patient which may affect reactions to the pill. To help eliminate the role of patient expectation during testing, a placebo (or fake medicine) is used on subjects in a control group. A researcher’s expectations may also affect results. Therefore, double-blind studies are often used. In such studies, neither the researcher nor the participants know which group is which. 2- Assume that researchers find that people’s memories are sharpest right after they’ve eaten lunch. What hidden variables may have affected these results? Other variables that may play a role (hidden variables): Time of Day Type of food eaten Meaning of “lunch” - May mean “time away from work” - Subjective State of feeling free (not food) helps memory. Socializing at lunch may make a person more alert and then able to take-in more info and retain it longer. 3- How might you use the scientific method to study factors that affect obedience? Devise a simple study, and identify the following: hypothesis, subjects, independent variable, dependent variable, experimental group, and control group. Hypothesis: If employee fears/believes they will be punished if they don’t follow directives, then they will be obedient to directives of bosses/people in “higher” positions than themselves in the workplace. Subjects: Employees Independent Variable: Directive given with harsh consequences for not following through. Dependent Variable: Obedience (Level of) Experimental Group: Group with “harsh” bosses (very by the book; give extreme consequences) present during their work day. -

Incidence and Secondary Transmission of SARS-Cov-2 Infections in Schools
Prepublication Release Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools Kanecia O. Zimmerman, MD; Ibukunoluwa C. Akinboyo, MD; M. Alan Brookhart, PhD; Angelique E. Boutzoukas, MD; Kathleen McGann, MD; Michael J. Smith, MD, MSCE; Gabriela Maradiaga Panayotti, MD; Sarah C. Armstrong, MD; Helen Bristow, MPH; Donna Parker, MPH; Sabrina Zadrozny, PhD; David J. Weber, MD, MPH; Daniel K. Benjamin, Jr., MD, PhD; for The ABC Science Collaborative DOI: 10.1542/peds.2020-048090 Journal: Pediatrics Article Type: Regular Article Citation: Zimmerman KO, Akinboyo IC, Brookhart A, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. 2021; doi: 10.1542/peds.2020- 048090 This is a prepublication version of an article that has undergone peer review and been accepted for publication but is not the final version of record. This paper may be cited using the DOI and date of access. This paper may contain information that has errors in facts, figures, and statements, and will be corrected in the final published version. The journal is providing an early version of this article to expedite access to this information. The American Academy of Pediatrics, the editors, and authors are not responsible for inaccurate information and data described in this version. Downloaded from©2021 www.aappublications.org/news American Academy by of guest Pediatrics on September 27, 2021 Prepublication Release Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools Kanecia O. Zimmerman, MD1,2,3; Ibukunoluwa C. Akinboyo, MD1,2; M. Alan Brookhart, PhD4; Angelique E. Boutzoukas, MD1,2; Kathleen McGann, MD2; Michael J. -

Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests Document Issued On: March 13, 2007
Guidance for Industry and FDA Staff Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests Document issued on: March 13, 2007 The draft of this document was issued on March 12, 2003. For questions regarding this document, contact Kristen Meier at 240-276-3060, or send an e-mail to [email protected]. U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health Diagnostic Devices Branch Division of Biostatistics Office of Surveillance and Biometrics Contains Nonbinding Recommendations Preface Public Comment Written comments and suggestions may be submitted at any time for Agency consideration to the Division of Dockets Management, Food and Drug Administration, 5630 Fishers Lane, Room 1061, (HFA-305), Rockville, MD, 20852. Alternatively, electronic comments may be submitted to http://www.fda.gov/dockets/ecomments. When submitting comments, please refer to Docket No. 2003D-0044. Comments may not be acted upon by the Agency until the document is next revised or updated. Additional Copies Additional copies are available from the Internet at: http://www.fda.gov/cdrh/osb/guidance/1620.pdf. You may also send an e-mail request to [email protected] to receive an electronic copy of the guidance or send a fax request to 240-276-3151 to receive a hard copy. Please use the document number 1620 to identify the guidance you are requesting. Contains Nonbinding Recommendations Table of Contents 1. Background....................................................................................................4 -

Estimated HIV Incidence and Prevalence in the United States
Volume 26, Number 1 Estimated HIV Incidence and Prevalence in the United States, 2015–2019 This issue of the HIV Surveillance Supplemental Report is published by the Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, Georgia. Estimates are presented for the incidence and prevalence of HIV infection among adults and adolescents (aged 13 years and older) based on data reported to CDC through December 2020. The HIV Surveillance Supplemental Report is not copyrighted and may be used and reproduced without permission. Citation of the source is, however, appreciated. Suggested citation Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021;26(No. 1). http://www.cdc.gov/ hiv/library/reports/hiv-surveillance.html. Published May 2021. Accessed [date]. On the Web: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html Confidential information, referrals, and educational material on HIV infection CDC-INFO 1-800-232-4636 (in English, en Español) 1-888-232-6348 (TTY) http://wwwn.cdc.gov/dcs/ContactUs/Form Acknowledgments Publication of this report was made possible by the contributions of the state and territorial health departments and the HIV surveillance programs that provided surveillance data to CDC. This report was prepared by the following staff and contractors of the Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC: Laurie Linley, Anna Satcher Johnson, Ruiguang Song, Sherry Hu, Baohua Wu, H. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Case Series: COVID-19 Infection Causing New-Onset Diabetes Mellitus?
Endocrinology & Metabolism International Journal Case Series Open Access Case series: COVID-19 infection causing new-onset diabetes mellitus? Abstract Volume 9 Issue 1 - 2021 Objectives: This paper seeks to explore the hypothesis of the potential diabetogenic effect 1 2 of SARS-COV-2 (Severe Acute respiratory syndrome coronavirus). Rujuta Katkar, Narasa Raju Madam 1Consultant Endocrinologist at Yuma Regional Medical Center, Case series presentation: We present a case series of observation among 8 patients of age USA 2 group ranging from 34 to 74 years with a BMI range of 26.61 to 53.21 Kilogram/square Hospitalist at Yuma Regional Medical Center, USA meters that developed new-onset diabetes after COVID-19 infection. Correspondence: Rujuta Katkar, MD, Consultant Severe Acute Respiratory Syndrome Coronavirus (SARS-COV-2), commonly known as Endocrinologist at Yuma Regional Medical Center, 2851 S Coronavirus or COVID-19(Coronavirus infectious disease), gains entry into the cells by Avenue B, Bldg.20, Yuma, AZ-85364, USA, Tel 2034359976, binding to the Angiotensin-converting enzyme-2(ACE-2) receptors located in essential Email metabolic tissues including the pancreas, adipose tissue, small intestine, and kidneys. Received: March 25, 2021 | Published: April 02, 2021 The evidence reviewed from the scientific literature describes how ACE 2 receptors play a role in the pathogenesis of diabetes and the plausible interaction of SARS-COV-2 with ACE 2 receptors in metabolic organs and tissues. Conclusion: The 8 patients without a past medical history of diabetes admitted with COVID-19 infection developed new-onset diabetes mellitus due to plausible interaction of SARS-COV-2 with ACE 2 receptors. -

Title: a TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF
1 Title: A TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF EXPLAINS 2 ATTENUATION OF HIV-1 IN UGANDA 3 Short title: EVOLUTION OF VIRULENCE IN HIV 4 François Blanquart1, Mary Kate Grabowski2, Joshua Herbeck3, Fred Nalugoda4, David 5 Serwadda4,5, Michael A. Eller6, 7, Merlin L. Robb6,7, Ronald Gray2,4, Godfrey Kigozi4, Oliver 6 Laeyendecker8, Katrina A. Lythgoe1,9, Gertrude Nakigozi4, Thomas C. Quinn8, Steven J. 7 Reynolds8, Maria J. Wawer2, Christophe Fraser1 8 1. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease 9 Epidemiology, School of Public Health, Imperial College London, United Kingdom 10 2. Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, 11 Baltimore, MD, USA 12 3. International Clinical Research Center, Department of Global Health, University of 13 Washington, Seattle, WA, USA 14 4. Rakai Health Sciences Program, Entebbe, Uganda 15 5. School of Public Health, Makerere University, Kampala, Uganda 16 6. U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, 17 MD, USA 18 7. Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA 19 8. Laboratory of Immunoregulation, Division of Intramural Research, National Institute of 20 Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA 21 9. Department of Zoology, University of Oxford, United Kingdom 22 23 Abstract 24 Evolutionary theory hypothesizes that intermediate virulence maximizes pathogen fitness as 25 a result of a trade-off between virulence and transmission, but empirical evidence remains scarce. 26 We bridge this gap using data from a large and long-standing HIV-1 prospective cohort, in 27 Uganda.