Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
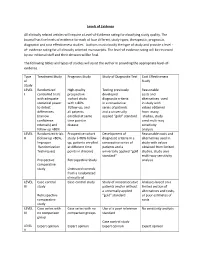
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

The Magic of Randomization Versus the Myth of Real-World Evidence
The new england journal of medicine Sounding Board The Magic of Randomization versus the Myth of Real-World Evidence Rory Collins, F.R.S., Louise Bowman, M.D., F.R.C.P., Martin Landray, Ph.D., F.R.C.P., and Richard Peto, F.R.S. Nonrandomized observational analyses of large safety and efficacy because the potential biases electronic patient databases are being promoted with respect to both can be appreciable. For ex- as an alternative to randomized clinical trials as ample, the treatment that is being assessed may a source of “real-world evidence” about the effi- well have been provided more or less often to cacy and safety of new and existing treatments.1-3 patients who had an increased or decreased risk For drugs or procedures that are already being of various health outcomes. Indeed, that is what used widely, such observational studies may in- would be expected in medical practice, since both volve exposure of large numbers of patients. the severity of the disease being treated and the Consequently, they have the potential to detect presence of other conditions may well affect the rare adverse effects that cannot plausibly be at- choice of treatment (often in ways that cannot be tributed to bias, generally because the relative reliably quantified). Even when associations of risk is large (e.g., Reye’s syndrome associated various health outcomes with a particular treat- with the use of aspirin, or rhabdomyolysis as- ment remain statistically significant after adjust- sociated with the use of statin therapy).4 Non- ment for all the known differences between pa- randomized clinical observation may also suf- tients who received it and those who did not fice to detect large beneficial effects when good receive it, these adjusted associations may still outcomes would not otherwise be expected (e.g., reflect residual confounding because of differ- control of diabetic ketoacidosis with insulin treat- ences in factors that were assessed only incom- ment, or the rapid shrinking of tumors with pletely or not at all (and therefore could not be chemotherapy). -

Adaptive Clinical Trials: an Introduction
Adaptive clinical trials: an introduction What are the advantages and disadvantages of adaptive clinical trial designs? How and why were Introduction adaptive clinical Adaptive clinical trial design is trials developed? becoming a hot topic in healthcare research, with some researchers In 2004, the FDA published a report arguing that adaptive trials have the on the problems faced by the potential to get new drugs to market scientific community in developing quicker. In this article, we explain new medical treatments.2 The what adaptive trials are and why they report highlighted that the pace of were developed, and we explore both innovation in biomedical science is the advantages of adaptive designs outstripping the rate of advances and the concerns being raised by in the available technologies and some in the healthcare community. tools for evaluating new treatments. Outdated tools are being used to assess new treatments and there What are adaptive is a critical need to improve the effectiveness and efficiency of clinical trials? clinical trials.2 The current process for developing Adaptive clinical trials enable new treatments is expensive, takes researchers to change an aspect a long time and in some cases, the of a trial design at an interim development process has to be assessment, while controlling stopped after significant amounts the rate of type 1 errors.1 Interim of time and resources have been assessments can help to determine invested.2 In 2006, the FDA published whether a trial design is the most a “Critical Path Opportunities -

(Meta-Analyses of Observational Studies in Epidemiology) Checklist
MOOSE (Meta-analyses Of Observational Studies in Epidemiology) Checklist A reporting checklist for Authors, Editors, and Reviewers of Meta-analyses of Observational Studies. You must report the page number in your manuscript where you consider each of the items listed in this checklist. If you have not included this information, either revise your manuscript accordingly before submitting or note N/A. Reporting Criteria Reported (Yes/No) Reported on Page No. Reporting of Background Problem definition Hypothesis statement Description of Study Outcome(s) Type of exposure or intervention used Type of study design used Study population Reporting of Search Strategy Qualifications of searchers (eg, librarians and investigators) Search strategy, including time period included in the synthesis and keywords Effort to include all available studies, including contact with authors Databases and registries searched Search software used, name and version, including special features used (eg, explosion) Use of hand searching (eg, reference lists of obtained articles) List of citations located and those excluded, including justification Method for addressing articles published in languages other than English Method of handling abstracts and unpublished studies Description of any contact with authors Reporting of Methods Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested Rationale for the selection and coding of data (eg, sound clinical principles or convenience) Documentation of how data were classified -

Epidemiology and Biostatistics (EPBI) 1
Epidemiology and Biostatistics (EPBI) 1 Epidemiology and Biostatistics (EPBI) Courses EPBI 2219. Biostatistics and Public Health. 3 Credit Hours. This course is designed to provide students with a solid background in applied biostatistics in the field of public health. Specifically, the course includes an introduction to the application of biostatistics and a discussion of key statistical tests. Appropriate techniques to measure the extent of disease, the development of disease, and comparisons between groups in terms of the extent and development of disease are discussed. Techniques for summarizing data collected in samples are presented along with limited discussion of probability theory. Procedures for estimation and hypothesis testing are presented for means, for proportions, and for comparisons of means and proportions in two or more groups. Multivariable statistical methods are introduced but not covered extensively in this undergraduate course. Public Health majors, minors or students studying in the Public Health concentration must complete this course with a C or better. Level Registration Restrictions: May not be enrolled in one of the following Levels: Graduate. Repeatability: This course may not be repeated for additional credits. EPBI 2301. Public Health without Borders. 3 Credit Hours. Public Health without Borders is a course that will introduce you to the world of disease detectives to solve public health challenges in glocal (i.e., global and local) communities. You will learn about conducting disease investigations to support public health actions relevant to affected populations. You will discover what it takes to become a field epidemiologist through hands-on activities focused on promoting health and preventing disease in diverse populations across the globe. -

A Guide to Systematic Review and Meta-Analysis of Prediction Model Performance
RESEARCH METHODS AND REPORTING A guide to systematic review and meta-analysis of prediction model performance Thomas P A Debray,1,2 Johanna A A G Damen,1,2 Kym I E Snell,3 Joie Ensor,3 Lotty Hooft,1,2 Johannes B Reitsma,1,2 Richard D Riley,3 Karel G M Moons1,2 1Cochrane Netherlands, Validation of prediction models is diagnostic test accuracy studies. Compared to therapeu- University Medical Center tic intervention and diagnostic test accuracy studies, Utrecht, PO Box 85500 Str highly recommended and increasingly there is limited guidance on the conduct of systematic 6.131, 3508 GA Utrecht, Netherlands common in the literature. A systematic reviews and meta-analysis of primary prognosis studies. 2Julius Center for Health review of validation studies is therefore A common aim of primary prognostic studies con- Sciences and Primary Care, cerns the development of so-called prognostic predic- University Medical Center helpful, with meta-analysis needed to tion models or indices. These models estimate the Utrecht, PO Box 85500 Str 6.131, 3508 GA Utrecht, summarise the predictive performance individualised probability or risk that a certain condi- Netherlands of the model being validated across tion will occur in the future by combining information 3Research Institute for Primary from multiple prognostic factors from an individual. Care and Health Sciences, Keele different settings and populations. This Unfortunately, there is often conflicting evidence about University, Staffordshire, UK article provides guidance for the predictive performance of developed prognostic Correspondence to: T P A Debray [email protected] researchers systematically reviewing prediction models. For this reason, there is a growing Additional material is published demand for evidence synthesis of (external validation) online only. -

Is It Worthwhile Including Observational Studies in Systematic Reviews of Effectiveness?
CRD_mcdaid05_Poster.qxd 13/6/05 5:12 pm Page 1 Is it worthwhile including observational studies in systematic reviews of effectiveness? The experience from a review of treatments for childhood retinoblastoma Catriona Mc Daid, Suzanne Hartley, Anne-Marie Bagnall, Gill Ritchie, Kate Light, Rob Riemsma Centre for Reviews and Dissemination, University of York, UK Background • Overall there were considerable problems with quality Figure 1: Mapping of included studies (see Table). Without randomised allocation there was a Retinoblastoma is a rare malignant tumour of the retina and high risk of selection bias in all studies. Studies were also usually occurs in children under two years old. It is an susceptible to detection and performance bias, with the aggressive tumour that can lead to loss of vision and, in retrospective studies particularly susceptible as they were extreme cases, death. The prognoses for vision and survival less likely to have a study protocol specifying the have significantly improved with the development of more intervention and outcome assessments. timely diagnosis and improved treatment methods. Important • Due to the considerable limitations of the evidence clinical factors associated with prognosis are age and stage of identified, it was not possible to make meaningful and disease at diagnosis. Patients with the hereditary form of robust conclusions about the relative effectiveness of retinoblastoma may be predisposed to significant long-term different treatment approaches for childhood complications. retinoblastoma. Historically, enucleation was the standard treatment for unilateral retinoblastoma. In bilateral retinoblastoma, the eye ■ ■ ■ with the most advanced tumour was commonly removed and EBRT Chemotherapy EBRT with Chemotherapy the contralateral eye treated with external beam radiotherapy ■ Enucleation ■ Local Treatments (EBRT). -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

How to Get Started with a Systematic Review in Epidemiology: an Introductory Guide for Early Career Researchers
Denison et al. Archives of Public Health 2013, 71:21 http://www.archpublichealth.com/content/71/1/21 ARCHIVES OF PUBLIC HEALTH METHODOLOGY Open Access How to get started with a systematic review in epidemiology: an introductory guide for early career researchers Hayley J Denison1*†, Richard M Dodds1,2†, Georgia Ntani1†, Rachel Cooper3†, Cyrus Cooper1†, Avan Aihie Sayer1,2† and Janis Baird1† Abstract Background: Systematic review is a powerful research tool which aims to identify and synthesize all evidence relevant to a research question. The approach taken is much like that used in a scientific experiment, with high priority given to the transparency and reproducibility of the methods used and to handling all evidence in a consistent manner. Early career researchers may find themselves in a position where they decide to undertake a systematic review, for example it may form part or all of a PhD thesis. Those with no prior experience of systematic review may need considerable support and direction getting started with such a project. Here we set out in simple terms how to get started with a systematic review. Discussion: Advice is given on matters such as developing a review protocol, searching using databases and other methods, data extraction, risk of bias assessment and data synthesis including meta-analysis. Signposts to further information and useful resources are also given. Conclusion: A well-conducted systematic review benefits the scientific field by providing a summary of existing evidence and highlighting unanswered questions. For the individual, undertaking a systematic review is also a great opportunity to improve skills in critical appraisal and in synthesising evidence. -

Guidelines for Reporting Meta-Epidemiological Methodology Research
EBM Primer Evid Based Med: first published as 10.1136/ebmed-2017-110713 on 12 July 2017. Downloaded from Guidelines for reporting meta-epidemiological methodology research Mohammad Hassan Murad, Zhen Wang 10.1136/ebmed-2017-110713 Abstract The goal is generally broad but often focuses on exam- Published research should be reported to evidence users ining the impact of certain characteristics of clinical studies on the observed effect, describing the distribu- Evidence-Based Practice with clarity and transparency that facilitate optimal tion of research evidence in a specific setting, exam- Center, Mayo Clinic, Rochester, appraisal and use of evidence and allow replication Minnesota, USA by other researchers. Guidelines for such reporting ining heterogeneity and exploring its causes, identifying are available for several types of studies but not for and describing plausible biases and providing empirical meta-epidemiological methodology studies. Meta- evidence for hypothesised associations. Unlike classic Correspondence to: epidemiological studies adopt a systematic review epidemiology, the unit of analysis for meta-epidemio- Dr Mohammad Hassan Murad, or meta-analysis approach to examine the impact logical studies is a study, not a patient. The outcomes Evidence-based Practice Center, of certain characteristics of clinical studies on the of meta-epidemiological studies are usually not clinical Mayo Clinic, 200 First Street 6–8 observed effect and provide empirical evidence for outcomes. SW, Rochester, MN 55905, USA; hypothesised associations. The unit of analysis in meta- In this guide, we adapt the items used in the PRISMA murad. mohammad@ mayo. edu 9 epidemiological studies is a study, not a patient. The statement for reporting systematic reviews and outcomes of meta-epidemiological studies are usually meta-analysis to fit the setting of meta- epidemiological not clinical outcomes. -

Adaptive Designs in Clinical Trials: Why Use Them, and How to Run and Report Them Philip Pallmann1* , Alun W
Pallmann et al. BMC Medicine (2018) 16:29 https://doi.org/10.1186/s12916-018-1017-7 CORRESPONDENCE Open Access Adaptive designs in clinical trials: why use them, and how to run and report them Philip Pallmann1* , Alun W. Bedding2, Babak Choodari-Oskooei3, Munyaradzi Dimairo4,LauraFlight5, Lisa V. Hampson1,6, Jane Holmes7, Adrian P. Mander8, Lang’o Odondi7, Matthew R. Sydes3,SofíaS.Villar8, James M. S. Wason8,9, Christopher J. Weir10, Graham M. Wheeler8,11, Christina Yap12 and Thomas Jaki1 Abstract Adaptive designs can make clinical trials more flexible by utilising results accumulating in the trial to modify the trial’s course in accordance with pre-specified rules. Trials with an adaptive design are often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money, and might require fewer participants. Adaptive designs can be applied across all phases of clinical research, from early-phase dose escalation to confirmatory trials. The pace of the uptake of adaptive designs in clinical research, however, has remained well behind that of the statistical literature introducing new methods and highlighting their potential advantages. We speculate that one factor contributing to this is that the full range of adaptations available to trial designs, as well as their goals, advantages and limitations, remains unfamiliar to many parts of the clinical community. Additionally, the term adaptive design has been misleadingly used as an all-encompassing label to refer to certain methods that could be deemed controversial or that have been inadequately implemented. We believe that even if the planning and analysis of a trial is undertaken by an expert statistician, it is essential that the investigators understand the implications of using an adaptive design, for example, what the practical challenges are, what can (and cannot) be inferred from the results of such a trial, and how to report and communicate the results. -

Top Ten Cool Things About 250B Exam 1
Cohort Studies Madhukar Pai, MD, PhD McGill University Montreal 1 Cohort: origins of the word http://www.caerleon.net/ 2 Introduction • Measurement of the occurrence of events over time is a central goal of epidemiologic research • Regardless of any particular study design or hypothesis, interest is ultimately in the disease or outcome-causing properties of factors that are antecedent to the disease or outcome. • All study designs (including case control and cross- sectional studies) are played out in some populations over time (either well defined cohorts or not) – They differ in how they acknowledge time and how they sample exposed and non-exposed as these groups develop disease over time 3 All the action happens within a “sea of person-time” in which events occur 4 Morgenstern IJE 1980 Cohort studies Intuitive approach to studying disease incidence and risk factors: 1. Start with a population at risk 2. Measure exposures and covariates at baseline 3. Follow-up the cohort over time with a) Surveillance for events or b) re-examination 4. Keep track of attrition, withdrawals, drop-outs and competing risks 5. For covariates that change over time, measure them again during follow up 6. Compare event rates in people with and without exposures of interest - Incidence Density Ratio (IDR) is the most natural and appropriate measure of effect - Adjust for confounders and compute adjusted IDR - Look for effect measure modification, if appropriate 5 Cohort studies • Can be large or small • Can be long or short duration • Can be simple or elaborate • Can look at multiple exposures and multiple outcomes • Can look at changes in exposures over time • For rare outcomes need many people and/or lengthy follow- up • Are usually very expensive because of the numbers and follow-up requirements • But once a cohort is established, can sustain research productivity for a long, long time! 6 Cohort: keeping track of people 7 Szklo & Nieto.