Epidemiologic Study Designs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
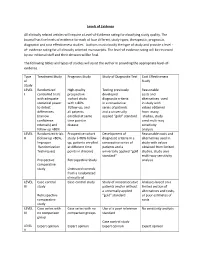
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

FDA Oncology Experience in Innovative Adaptive Trial Designs
Innovative Adaptive Trial Designs Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Office of Biostatistics, CDER, FDA 9/3/2015 Sridhara - Ovarian cancer workshop 1 Fixed Sample Designs • Patient population, disease assessments, treatment, sample size, hypothesis to be tested, primary outcome measure - all fixed • No change in the design features during the study Adaptive Designs • A study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (interim data) from subjects in the study 9/3/2015 Sridhara - Ovarian cancer workshop 2 Bayesian Designs • In the Bayesian paradigm, the parameter measuring treatment effect is regarded as a random variable • Bayesian inference is based on the posterior distribution (Bayes’ Rule – updated based on observed data) – Outcome adaptive • By definition adaptive design 9/3/2015 Sridhara - Ovarian cancer workshop 3 Adaptive Designs (Frequentist or Bayesian) • Allows for planned design modifications • Modifications based on data accrued in the trial up to the interim time • Unblinded or blinded interim results • Control probability of false positive rate for multiple options • Control operational bias • Assumes independent increments of information 9/3/2015 Sridhara - Ovarian cancer workshop 4 Enrichment Designs – Prognostic or Predictive • Untargeted or All comers design: – post-hoc enrichment, prospective-retrospective designs – Marker evaluation after randomization (example: KRAS in cetuximab -

FORMULAS from EPIDEMIOLOGY KEPT SIMPLE (3E) Chapter 3: Epidemiologic Measures
FORMULAS FROM EPIDEMIOLOGY KEPT SIMPLE (3e) Chapter 3: Epidemiologic Measures Basic epidemiologic measures used to quantify: • frequency of occurrence • the effect of an exposure • the potential impact of an intervention. Epidemiologic Measures Measures of disease Measures of Measures of potential frequency association impact (“Measures of Effect”) Incidence Prevalence Absolute measures of Relative measures of Attributable Fraction Attributable Fraction effect effect in exposed cases in the Population Incidence proportion Incidence rate Risk difference Risk Ratio (Cumulative (incidence density, (Incidence proportion (Incidence Incidence, Incidence hazard rate, person- difference) Proportion Ratio) Risk) time rate) Incidence odds Rate Difference Rate Ratio (Incidence density (Incidence density difference) ratio) Prevalence Odds Ratio Difference Macintosh HD:Users:buddygerstman:Dropbox:eks:formula_sheet.doc Page 1 of 7 3.1 Measures of Disease Frequency No. of onsets Incidence Proportion = No. at risk at beginning of follow-up • Also called risk, average risk, and cumulative incidence. • Can be measured in cohorts (closed populations) only. • Requires follow-up of individuals. No. of onsets Incidence Rate = ∑person-time • Also called incidence density and average hazard. • When disease is rare (incidence proportion < 5%), incidence rate ≈ incidence proportion. • In cohorts (closed populations), it is best to sum individual person-time longitudinally. It can also be estimated as Σperson-time ≈ (average population size) × (duration of follow-up). Actuarial adjustments may be needed when the disease outcome is not rare. • In an open populations, Σperson-time ≈ (average population size) × (duration of follow-up). Examples of incidence rates in open populations include: births Crude birth rate (per m) = × m mid-year population size deaths Crude mortality rate (per m) = × m mid-year population size deaths < 1 year of age Infant mortality rate (per m) = × m live births No. -

Incidence and Secondary Transmission of SARS-Cov-2 Infections in Schools
Prepublication Release Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools Kanecia O. Zimmerman, MD; Ibukunoluwa C. Akinboyo, MD; M. Alan Brookhart, PhD; Angelique E. Boutzoukas, MD; Kathleen McGann, MD; Michael J. Smith, MD, MSCE; Gabriela Maradiaga Panayotti, MD; Sarah C. Armstrong, MD; Helen Bristow, MPH; Donna Parker, MPH; Sabrina Zadrozny, PhD; David J. Weber, MD, MPH; Daniel K. Benjamin, Jr., MD, PhD; for The ABC Science Collaborative DOI: 10.1542/peds.2020-048090 Journal: Pediatrics Article Type: Regular Article Citation: Zimmerman KO, Akinboyo IC, Brookhart A, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. 2021; doi: 10.1542/peds.2020- 048090 This is a prepublication version of an article that has undergone peer review and been accepted for publication but is not the final version of record. This paper may be cited using the DOI and date of access. This paper may contain information that has errors in facts, figures, and statements, and will be corrected in the final published version. The journal is providing an early version of this article to expedite access to this information. The American Academy of Pediatrics, the editors, and authors are not responsible for inaccurate information and data described in this version. Downloaded from©2021 www.aappublications.org/news American Academy by of guest Pediatrics on September 27, 2021 Prepublication Release Incidence and Secondary Transmission of SARS-CoV-2 Infections in Schools Kanecia O. Zimmerman, MD1,2,3; Ibukunoluwa C. Akinboyo, MD1,2; M. Alan Brookhart, PhD4; Angelique E. Boutzoukas, MD1,2; Kathleen McGann, MD2; Michael J. -

Estimated HIV Incidence and Prevalence in the United States
Volume 26, Number 1 Estimated HIV Incidence and Prevalence in the United States, 2015–2019 This issue of the HIV Surveillance Supplemental Report is published by the Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, Georgia. Estimates are presented for the incidence and prevalence of HIV infection among adults and adolescents (aged 13 years and older) based on data reported to CDC through December 2020. The HIV Surveillance Supplemental Report is not copyrighted and may be used and reproduced without permission. Citation of the source is, however, appreciated. Suggested citation Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021;26(No. 1). http://www.cdc.gov/ hiv/library/reports/hiv-surveillance.html. Published May 2021. Accessed [date]. On the Web: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html Confidential information, referrals, and educational material on HIV infection CDC-INFO 1-800-232-4636 (in English, en Español) 1-888-232-6348 (TTY) http://wwwn.cdc.gov/dcs/ContactUs/Form Acknowledgments Publication of this report was made possible by the contributions of the state and territorial health departments and the HIV surveillance programs that provided surveillance data to CDC. This report was prepared by the following staff and contractors of the Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC: Laurie Linley, Anna Satcher Johnson, Ruiguang Song, Sherry Hu, Baohua Wu, H. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Disease Incidence, Prevalence and Disability
Part 3 Disease incidence, prevalence and disability 9. How many people become sick each year? 28 10. Cancer incidence by site and region 29 11. How many people are sick at any given time? 31 12. Prevalence of moderate and severe disability 31 13. Leading causes of years lost due to disability in 2004 36 World Health Organization 9. How many people become sick each such as diarrhoeal disease or malaria, it is common year? for individuals to be infected repeatedly and have several episodes. For such conditions, the number The “incidence” of a condition is the number of new given in the table is the number of disease episodes, cases in a period of time – usually one year (Table 5). rather than the number of individuals affected. For most conditions in this table, the figure given is It is important to remember that the incidence of the number of individuals who developed the illness a disease or condition measures how many people or problem in 2004. However, for some conditions, are affected by it for the first time over a period of Table 5: Incidence (millions) of selected conditions by WHO region, 2004 Eastern The Mediter- South- Western World Africa Americas ranean Europe East Asia Pacific Tuberculosisa 7.8 1.4 0.4 0.6 0.6 2.8 2.1 HIV infectiona 2.8 1.9 0.2 0.1 0.2 0.2 0.1 Diarrhoeal diseaseb 4 620.4 912.9 543.1 424.9 207.1 1 276.5 1 255.9 Pertussisb 18.4 5.2 1.2 1.6 0.7 7.5 2.1 Measlesa 27.1 5.3 0.0e 1.0 0.2 17.4 3.3 Tetanusa 0.3 0.1 0.0 0.1 0.0 0.1 0.0 Meningitisb 0.7 0.3 0.1 0.1 0.0 0.2 0.1 Malariab 241.3 203.9 2.9 8.6 0.0 23.3 2.7 -

Title: a TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF
1 Title: A TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF EXPLAINS 2 ATTENUATION OF HIV-1 IN UGANDA 3 Short title: EVOLUTION OF VIRULENCE IN HIV 4 François Blanquart1, Mary Kate Grabowski2, Joshua Herbeck3, Fred Nalugoda4, David 5 Serwadda4,5, Michael A. Eller6, 7, Merlin L. Robb6,7, Ronald Gray2,4, Godfrey Kigozi4, Oliver 6 Laeyendecker8, Katrina A. Lythgoe1,9, Gertrude Nakigozi4, Thomas C. Quinn8, Steven J. 7 Reynolds8, Maria J. Wawer2, Christophe Fraser1 8 1. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease 9 Epidemiology, School of Public Health, Imperial College London, United Kingdom 10 2. Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, 11 Baltimore, MD, USA 12 3. International Clinical Research Center, Department of Global Health, University of 13 Washington, Seattle, WA, USA 14 4. Rakai Health Sciences Program, Entebbe, Uganda 15 5. School of Public Health, Makerere University, Kampala, Uganda 16 6. U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, 17 MD, USA 18 7. Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA 19 8. Laboratory of Immunoregulation, Division of Intramural Research, National Institute of 20 Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA 21 9. Department of Zoology, University of Oxford, United Kingdom 22 23 Abstract 24 Evolutionary theory hypothesizes that intermediate virulence maximizes pathogen fitness as 25 a result of a trade-off between virulence and transmission, but empirical evidence remains scarce. 26 We bridge this gap using data from a large and long-standing HIV-1 prospective cohort, in 27 Uganda. -

Catalogue of Clinical Trials and Cohort Studies to Identify Biological
Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for recent HIV infection Final Report February 2010 This final report was prepared by Dr Joanne Micallef and Professor John Kaldor, The University of New South Wales, under subcontract with Family Health International, funded by the Bill and Melinda Gates Foundation under the grant titled “Development of Assays for Acute HIV Infection and Estimation and HIV Incidence in Population”. 1 Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Table of Contents Table of Contents .................................................................................................................................................................... 2 List of acronyms ...................................................................................................................................................................... 4 1 Introduction ..................................................................................................................................................................... 6 2 Methods ............................................................................................................................................................................ -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Incidence Density Sampling for Nested Case-Control Study Designs
Paper AS09 Incidence Density Sampling for Nested Case-Control Study Designs Quratul Ann, IQVIA, London, United Kingdom Rachel Tham, IQVIA, London, United Kingdom ABSTRACT The nested case-control design is commonly used in safety studies, as it is an efficient approach to an epidemiological investigation. When the design is implemented appropriately, it can yield unbiased estimates of relative risk; and in exchange for a small loss in precision, considerable improved efficiency and reduction in costs can be achieved. There are several ways of sampling controls to cases in a nested case-control design. The most sophisticated way is via incidence density sampling. Incidence density sampling matches cases to controls based on the dynamic risk set at the time of case occurrence, where the probability of control selection is proportional to the total person-time at risk. Incidence density sampling alleviates the rare disease assumption; however, it is rarely used due to its computational complexity. This paper presents a novel and simple Statistical Analysis System (SAS) program for incidence density sampling, which could minimise bias and introduce a more appropriate way of optimising the matching of controls to cases. INTRODUCTION Retrospective studies gather available previous exposure information for a clearly defined source population and the outcome event is determined for all members of the cohort4. Retrospective studies facilitate an efficient approach to draw inferences and determine the association between exposure and disease prevalence particularly for rare outcomes and with long-term disease conditions. Real-world data (RWD) are a valuable source of information for investigating health-related outcomes in human populations.