Cohort Studies Or Prospective and Retrospective Cohort Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
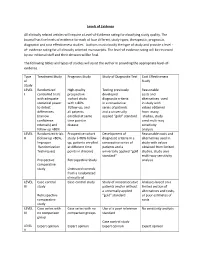
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

Basic Epidemiology for BPSU Studies
BPSU Study guidance – Basic epidemiology for BPSU studies Dr Simon Lenton on behalf of the Scientific Committee of the British Paediatric Surveillance Unit Updated 07 01 19 BPSU parent bodies: with support from: 1 Contents Introduction ................................................................................................................. 3 Concepts of disease development ..................................................................... 3 Public health surveillance ...................................................................................... 4 Epidemiology ............................................................................................................. 4 Descriptive epidemiology ...................................................................................... 5 Analytic epidemiology ............................................................................................ 5 Triangulation (cross verification) ........................................................................ 6 Capture-recapture .................................................................................................... 6 BPSU research design ............................................................................................. 6 BPSU resources .......................................................................................................... 9 Appendix .................................................................................................................... 10 References ................................................................................................................ -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Title: a TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF
1 Title: A TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF EXPLAINS 2 ATTENUATION OF HIV-1 IN UGANDA 3 Short title: EVOLUTION OF VIRULENCE IN HIV 4 François Blanquart1, Mary Kate Grabowski2, Joshua Herbeck3, Fred Nalugoda4, David 5 Serwadda4,5, Michael A. Eller6, 7, Merlin L. Robb6,7, Ronald Gray2,4, Godfrey Kigozi4, Oliver 6 Laeyendecker8, Katrina A. Lythgoe1,9, Gertrude Nakigozi4, Thomas C. Quinn8, Steven J. 7 Reynolds8, Maria J. Wawer2, Christophe Fraser1 8 1. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease 9 Epidemiology, School of Public Health, Imperial College London, United Kingdom 10 2. Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, 11 Baltimore, MD, USA 12 3. International Clinical Research Center, Department of Global Health, University of 13 Washington, Seattle, WA, USA 14 4. Rakai Health Sciences Program, Entebbe, Uganda 15 5. School of Public Health, Makerere University, Kampala, Uganda 16 6. U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, 17 MD, USA 18 7. Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA 19 8. Laboratory of Immunoregulation, Division of Intramural Research, National Institute of 20 Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA 21 9. Department of Zoology, University of Oxford, United Kingdom 22 23 Abstract 24 Evolutionary theory hypothesizes that intermediate virulence maximizes pathogen fitness as 25 a result of a trade-off between virulence and transmission, but empirical evidence remains scarce. 26 We bridge this gap using data from a large and long-standing HIV-1 prospective cohort, in 27 Uganda. -

Catalogue of Clinical Trials and Cohort Studies to Identify Biological
Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for recent HIV infection Final Report February 2010 This final report was prepared by Dr Joanne Micallef and Professor John Kaldor, The University of New South Wales, under subcontract with Family Health International, funded by the Bill and Melinda Gates Foundation under the grant titled “Development of Assays for Acute HIV Infection and Estimation and HIV Incidence in Population”. 1 Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Table of Contents Table of Contents .................................................................................................................................................................... 2 List of acronyms ...................................................................................................................................................................... 4 1 Introduction ..................................................................................................................................................................... 6 2 Methods ............................................................................................................................................................................ -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Download and Use
Shenkin et al. BMC Medicine (2019) 17:138 https://doi.org/10.1186/s12916-019-1367-9 RESEARCHARTICLE Open Access Delirium detection in older acute medical inpatients: a multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method Susan D. Shenkin1, Christopher Fox2, Mary Godfrey3, Najma Siddiqi4, Steve Goodacre5, John Young6, Atul Anand7, Alasdair Gray8, Janet Hanley9, Allan MacRaild8, Jill Steven8, Polly L. Black8, Zoë Tieges1, Julia Boyd10, Jacqueline Stephen10, Christopher J. Weir10 and Alasdair M. J. MacLullich1* Abstract Background: Delirium affects > 15% of hospitalised patients but is grossly underdetected, contributing to poor care. The 4 ‘A’s Test (4AT, www.the4AT.com) is a short delirium assessment tool designed for routine use without special training. Theprimaryobjectivewastoassesstheaccuracyofthe4ATfor delirium detection. The secondary objective was to compare the 4AT with another commonly used delirium assessment tool, the Confusion Assessment Method (CAM). Methods: This was a prospective diagnostic test accuracy study set in emergency departments or acute medical wards involving acute medical patients aged ≥ 70. All those without acutely life-threatening illness or coma were eligible. Patients underwent (1) reference standard delirium assessment based on DSM-IV criteria and (2) were randomised to either the index test (4AT, scores 0–12; prespecified score of > 3 considered positive) or the comparator (CAM; scored positive or negative), in a random order, using computer-generated pseudo-random numbers, stratified by study site, with block allocation. Reference standard and 4AT or CAM assessments were performed by pairs of independent raters blinded to the results of the other assessment. Results: Eight hundred forty-three individuals were randomised: 21 withdrew, 3 lost contact, 32 indeterminate diagnosis, 2 missing outcome, and 785 were included in the analysis. -

Top Ten Cool Things About 250B Exam 1
Cohort Studies Madhukar Pai, MD, PhD McGill University Montreal 1 Cohort: origins of the word http://www.caerleon.net/ 2 Introduction • Measurement of the occurrence of events over time is a central goal of epidemiologic research • Regardless of any particular study design or hypothesis, interest is ultimately in the disease or outcome-causing properties of factors that are antecedent to the disease or outcome. • All study designs (including case control and cross- sectional studies) are played out in some populations over time (either well defined cohorts or not) – They differ in how they acknowledge time and how they sample exposed and non-exposed as these groups develop disease over time 3 All the action happens within a “sea of person-time” in which events occur 4 Morgenstern IJE 1980 Cohort studies Intuitive approach to studying disease incidence and risk factors: 1. Start with a population at risk 2. Measure exposures and covariates at baseline 3. Follow-up the cohort over time with a) Surveillance for events or b) re-examination 4. Keep track of attrition, withdrawals, drop-outs and competing risks 5. For covariates that change over time, measure them again during follow up 6. Compare event rates in people with and without exposures of interest - Incidence Density Ratio (IDR) is the most natural and appropriate measure of effect - Adjust for confounders and compute adjusted IDR - Look for effect measure modification, if appropriate 5 Cohort studies • Can be large or small • Can be long or short duration • Can be simple or elaborate • Can look at multiple exposures and multiple outcomes • Can look at changes in exposures over time • For rare outcomes need many people and/or lengthy follow- up • Are usually very expensive because of the numbers and follow-up requirements • But once a cohort is established, can sustain research productivity for a long, long time! 6 Cohort: keeping track of people 7 Szklo & Nieto. -

Epidemiologic Study Designs
Epidemiologic Study Designs Jacky M Jennings, PhD, MPH Associate Professor Associate Director, General Pediatrics and Adolescent Medicine Director, Center for Child & Community Health Research (CCHR) Departments of Pediatrics & Epidemiology Johns Hopkins University Learning Objectives • Identify basic epidemiologic study designs and their frequent sequence of study • Recognize the basic components • Understand the advantages and disadvantages • Appropriately select a study design Research Question & Hypotheses Analytic Study Plan Design Basic Study Designs and their Hierarchy Clinical Observation Hypothesis Descriptive Study Case-Control Study Cohort Study Randomized Controlled Trial Systematic Review Causality Adapted from Gordis, 1996 MMWR Study Design in Epidemiology • Depends on: – The research question and hypotheses – Resources and time available for the study – Type of outcome of interest – Type of exposure of interest – Ethics Study Design in Epidemiology • Includes: – The research question and hypotheses – Measures and data quality – Time – Study population • Inclusion/exclusion criteria • Internal/external validity Epidemiologic Study Designs • Descriptive studies – Seeks to measure the frequency of disease and/or collect descriptive data on risk factors • Analytic studies – Tests a causal hypothesis about the etiology of disease • Experimental studies – Compares, for example, treatments EXPOSURE Cross-sectional OUTCOME EXPOSURE OUTCOME Case-Control EXPOSURE OUTCOME Cohort TIME Cross-sectional studies • Measure existing disease and current exposure levels at one point in time • Sample without knowledge of exposure or disease • Ex. Prevalence studies Cross-sectional studies • Advantages – Often early study design in a line of investigation – Good for hypothesis generation – Relatively easy, quick and inexpensive…depends on question – Examine multiple exposures or outcomes – Estimate prevalence of disease and exposures Cross-sectional studies • Disadvantages – Cannot infer causality – Prevalent vs. -

Systematic Review of the Prospective Cohort Studies on Meat Consumption and Colorectal Cancer Risk: a Meta-Analytical Approach1
Vol. 10, 439–446, May 2001 Cancer Epidemiology, Biomarkers & Prevention 439 Systematic Review of the Prospective Cohort Studies on Meat Consumption and Colorectal Cancer Risk: A Meta-Analytical Approach1 Manjinder S. Sandhu,2 Ian R. White, and confounded or modified by other factors cannot be Klim McPherson excluded. Department of Public Health and Primary Care, Institute of Public Health, University of Cambridge, Strangeways Research Laboratory, Cambridge, CB1 8RN [M. S. S.]; Medical Research Council Biostatistics Unit, Institute of Introduction Public Health, University of Cambridge, Cambridge, CB2 2SR [I. R. W.]; and The relation between meat consumption and colorectal cancer Cancer and Public Health Unit, London School of Hygiene and Tropical Medicine, London, WC1E 7HT [K. M.], United Kingdom risk remains controversial (1–10). Subsequent to the report of the National Academy of Sciences, “Diet and Health” (11), which implicated red meat as a causative factor in the etiology Abstract of colorectal cancer, two subsequent reports have reviewed the epidemiological evidence on meat and colorectal cancer risk (4, The relation between meat consumption and colorectal 3 cancer risk remains controversial. In this report, we 7). The report of the WCRF concluded: “The evidence shows quantitatively reviewed the prospective observational that red meat probably increases risk and processed meat pos- studies that have analyzed the relation between meat sibly increases risk of colorectal cancer” (7). The report from consumption and colorectal cancer. We conducted COMA judged that “there is moderately consistent evidence electronic searches of MEDLINE, EMBASE, and from cohort studies of a positive association between the con- CANCERLIT databases through to the end of June 1999 sumption of red or processed meat and risk of colorectal can- and manual searches of references from retrieved cer” (4). -

Overview of Epidemiological Study Designs
University of Massachusetts Medical School eScholarship@UMMS PEER Liberia Project UMass Medical School Collaborations in Liberia 2018-11 Overview of Epidemiological Study Designs Richard Ssekitoleko Yale University Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/liberia_peer Part of the Clinical Epidemiology Commons, Epidemiology Commons, Family Medicine Commons, Infectious Disease Commons, Medical Education Commons, and the Quantitative, Qualitative, Comparative, and Historical Methodologies Commons Repository Citation Ssekitoleko R. (2018). Overview of Epidemiological Study Designs. PEER Liberia Project. https://doi.org/ 10.13028/fp3z-mv12. Retrieved from https://escholarship.umassmed.edu/liberia_peer/5 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in PEER Liberia Project by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Overview of Epidemiological study Designs Richard Ssekitoleko Department of Global Health Yale University 1.1 Objectives • Understand the different epidemiological study types • Get to know what is involved in each type of study • Understand the strengths and Limitations of each study type Key terms • Population • Consists of all elements and is the group from which a sample is drawn • A sample is a subset of a population • Parameters • Summary data from a population • Statistics • Summary data from a sample • Validity • Extent to which a conclusion or statistic is well-founded and likely corresponds accurately to the parameter. Hierarchy of Evidence Study type Observational Interventional Descriptive Experiment Ecological Randomized Controlled Trial Cross-sectional Case-control Cohort Overview of Epidemiologic Study Designs Validity *anecdotes Cost Understanding What Physicians Mean: In my experience … once. -

Recommendations for Naming Study Design and Levels of Evidence
Recommendations for Naming Study Design and Levels of Evidence for Structured Abstracts in the Journal of Orthopedic & Sports Physical Therapy (JOSPT) Last revised 07-03-2008 The JOSPT as a journal that primarily focuses on clinically based research, attempts to assist readers in understanding the quality of research evidence in its published manuscripts by using a structured abstract that includes information on the “study design” and the classification of the “level of evidence.” While no single piece of information or classification can signify the quality and clinical relevance of published studies, a consistent approach to communicating this information is a step towards assisting our readers in evaluating new knowledge. Outline of the structured abstract for research reports published in JOSPT Study design Objectives Background Methods and Measures Results Conclusions Level of Evidence Suggestions for Naming Study Designs We recommend that authors attempt to use the following terminology when naming their research designs. Use of consistent terminology will make it easier for readers to identify the nature of the study and will allow us to track the type of studies published more easily. We recognize that this list is not all-inclusive and that more appropriate descriptors might be suitable for some studies. In those cases, investigators are encouraged to pick the most appropriate descriptors for their study. These suggestions are provided as a means of encouraging consistency where it would be both useful and informative and their use is expected where they do apply. Quantitative Clinical Study Categories include (Therapy, Prevention, Etiology, Harm, Prognosis, Diagnosis, Differential Diagnosis, Symptom Prevalence, Economic Analysis, and Decision Analysis).