Study Designs and Their Outcomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -
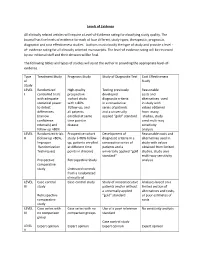
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

Meta-Analysis of Proportions
NCSS Statistical Software NCSS.com Chapter 456 Meta-Analysis of Proportions Introduction This module performs a meta-analysis of a set of two-group, binary-event studies. These studies have a treatment group (arm) and a control group. The results of each study may be summarized as counts in a 2-by-2 table. The program provides a complete set of numeric reports and plots to allow the investigation and presentation of the studies. The plots include the forest plot, radial plot, and L’Abbe plot. Both fixed-, and random-, effects models are available for analysis. Meta-Analysis refers to methods for the systematic review of a set of individual studies with the aim to combine their results. Meta-analysis has become popular for a number of reasons: 1. The adoption of evidence-based medicine which requires that all reliable information is considered. 2. The desire to avoid narrative reviews which are often misleading. 3. The desire to interpret the large number of studies that may have been conducted about a specific treatment. 4. The desire to increase the statistical power of the results be combining many small-size studies. The goals of meta-analysis may be summarized as follows. A meta-analysis seeks to systematically review all pertinent evidence, provide quantitative summaries, integrate results across studies, and provide an overall interpretation of these studies. We have found many books and articles on meta-analysis. In this chapter, we briefly summarize the information in Sutton et al (2000) and Thompson (1998). Refer to those sources for more details about how to conduct a meta- analysis. -

The Magic of Randomization Versus the Myth of Real-World Evidence
The new england journal of medicine Sounding Board The Magic of Randomization versus the Myth of Real-World Evidence Rory Collins, F.R.S., Louise Bowman, M.D., F.R.C.P., Martin Landray, Ph.D., F.R.C.P., and Richard Peto, F.R.S. Nonrandomized observational analyses of large safety and efficacy because the potential biases electronic patient databases are being promoted with respect to both can be appreciable. For ex- as an alternative to randomized clinical trials as ample, the treatment that is being assessed may a source of “real-world evidence” about the effi- well have been provided more or less often to cacy and safety of new and existing treatments.1-3 patients who had an increased or decreased risk For drugs or procedures that are already being of various health outcomes. Indeed, that is what used widely, such observational studies may in- would be expected in medical practice, since both volve exposure of large numbers of patients. the severity of the disease being treated and the Consequently, they have the potential to detect presence of other conditions may well affect the rare adverse effects that cannot plausibly be at- choice of treatment (often in ways that cannot be tributed to bias, generally because the relative reliably quantified). Even when associations of risk is large (e.g., Reye’s syndrome associated various health outcomes with a particular treat- with the use of aspirin, or rhabdomyolysis as- ment remain statistically significant after adjust- sociated with the use of statin therapy).4 Non- ment for all the known differences between pa- randomized clinical observation may also suf- tients who received it and those who did not fice to detect large beneficial effects when good receive it, these adjusted associations may still outcomes would not otherwise be expected (e.g., reflect residual confounding because of differ- control of diabetic ketoacidosis with insulin treat- ences in factors that were assessed only incom- ment, or the rapid shrinking of tumors with pletely or not at all (and therefore could not be chemotherapy). -

(Meta-Analyses of Observational Studies in Epidemiology) Checklist
MOOSE (Meta-analyses Of Observational Studies in Epidemiology) Checklist A reporting checklist for Authors, Editors, and Reviewers of Meta-analyses of Observational Studies. You must report the page number in your manuscript where you consider each of the items listed in this checklist. If you have not included this information, either revise your manuscript accordingly before submitting or note N/A. Reporting Criteria Reported (Yes/No) Reported on Page No. Reporting of Background Problem definition Hypothesis statement Description of Study Outcome(s) Type of exposure or intervention used Type of study design used Study population Reporting of Search Strategy Qualifications of searchers (eg, librarians and investigators) Search strategy, including time period included in the synthesis and keywords Effort to include all available studies, including contact with authors Databases and registries searched Search software used, name and version, including special features used (eg, explosion) Use of hand searching (eg, reference lists of obtained articles) List of citations located and those excluded, including justification Method for addressing articles published in languages other than English Method of handling abstracts and unpublished studies Description of any contact with authors Reporting of Methods Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested Rationale for the selection and coding of data (eg, sound clinical principles or convenience) Documentation of how data were classified -

Education Quarterly Reviews
Education Quarterly Reviews Allanson, Patricia E., and Notar, Charles E. (2020), Statistics as Measurement: 4 Scales/Levels of Measurement. In: Education Quarterly Reviews, Vol.3, No.3, 375-385. ISSN 2621-5799 DOI: 10.31014/aior.1993.03.03.146 The online version of this article can be found at: https://www.asianinstituteofresearch.org/ Published by: The Asian Institute of Research The Education Quarterly Reviews is an Open Access publication. It May be read, copied, and distributed free of charge according to the conditions of the Creative ComMons Attribution 4.0 International license. The Asian Institute of Research Education Quarterly Reviews is a peer-reviewed International Journal. The journal covers scholarly articles in the fields of education, linguistics, literature, educational theory, research, and methodologies, curriculum, elementary and secondary education, higher education, foreign language education, teaching and learning, teacher education, education of special groups, and other fields of study related to education. As the journal is Open Access, it ensures high visibility and the increase of citations for all research articles published. The Education Quarterly Reviews aiMs to facilitate scholarly work on recent theoretical and practical aspects of education. The Asian Institute of Research Education Quarterly Reviews Vol.3, No.3, 2020: 375-385 ISSN 2621-5799 Copyright © The Author(s). All Rights Reserved DOI: 10.31014/aior.1993.03.03.146 Statistics as Measurement: 4 Scales/Levels of Measurement 1 2 Patricia E. Allanson , Charles E. Notar 1 Liberty University 2 Jacksonville State University. EMail: [email protected] (EMeritus) Abstract This article discusses the basics of the “4 scales of MeasureMent” and how they are applicable to research or everyday tools of life. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Internal and External Validity: Can You Apply Research Study Results to Your Patients? Cecilia Maria Patino1,2,A, Juliana Carvalho Ferreira1,3,B
J Bras Pneumol. 2018;44(3):183-183 CONTINUING EDUCATION: http://dx.doi.org/10.1590/S1806-37562018000000164 SCIENTIFIC METHODOLOGY Internal and external validity: can you apply research study results to your patients? Cecilia Maria Patino1,2,a, Juliana Carvalho Ferreira1,3,b CLINICAL SCENARIO extent to which the results of a study are generalizable to patients in our daily practice, especially for the population In a multicenter study in France, investigators conducted that the sample is thought to represent. a randomized controlled trial to test the effect of prone vs. supine positioning ventilation on mortality among patients Lack of internal validity implies that the results of the with early, severe ARDS. They showed that prolonged study deviate from the truth, and, therefore, we cannot prone-positioning ventilation decreased 28-day mortality draw any conclusions; hence, if the results of a trial are [hazard ratio (HR) = 0.39; 95% CI: 0.25-0.63].(1) not internally valid, external validity is irrelevant.(2) Lack of external validity implies that the results of the trial may not apply to patients who differ from the study STUDY VALIDITY population and, consequently, could lead to low adoption The validity of a research study refers to how well the of the treatment tested in the trial by other clinicians. results among the study participants represent true fi ndings among similar individuals outside the study. INCREASING VALIDITY OF RESEARCH This concept of validity applies to all types of clinical STUDIES studies, including those about prevalence, associations, interventions, and diagnosis. The validity of a research To increase internal validity, investigators should ensure study includes two domains: internal and external validity. -

Survey Experiments
IU Workshop in Methods – 2019 Survey Experiments Testing Causality in Diverse Samples Trenton D. Mize Department of Sociology & Advanced Methodologies (AMAP) Purdue University Survey Experiments Page 1 Survey Experiments Page 2 Contents INTRODUCTION ............................................................................................................................................................................ 8 Overview .............................................................................................................................................................................. 8 What is a survey experiment? .................................................................................................................................... 9 What is an experiment?.............................................................................................................................................. 10 Independent and dependent variables ................................................................................................................. 11 Experimental Conditions ............................................................................................................................................. 12 WHY CONDUCT A SURVEY EXPERIMENT? ........................................................................................................................... 13 Internal, external, and construct validity .......................................................................................................... -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Conditional Process Analysis in Two-Instance Repeated-Measures Designs
Conditional Process Analysis in Two-Instance Repeated-Measures Designs Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Amanda Kay Montoya, M.S., M.A. Graduate Program in Department of Psychology The Ohio State University 2018 Dissertation Committee: Andrew F. Hayes, Advisor Jolynn Pek Paul de Boeck c Copyright by Amanda Kay Montoya 2018 Abstract Conditional process models are commonly used in many areas of psychol- ogy research as well as research in other academic fields (e.g., marketing, communication, and education). Conditional process models combine me- diation analysis and moderation analysis. Mediation analysis, sometimes called process analysis, investigates if an independent variable influences an outcome variable through a specific intermediary variable, sometimes called a mediator. Moderation analysis investigates if the relationship be- tween two variables depends on another. Conditional process models are very popular because they allow us to better understand how the processes we are interested in might vary depending on characteristics of different individuals, situations, and other moderating variables. Methodological developments in conditional process analysis have primarily focused on the analysis of data collected using between-subjects experimental designs or cross-sectional designs. However, another very common design is the two- instance repeated-measures design. A two-instance repeated-measures de- sign is one where each subject is measured twice; once in each of two instances. In the analysis discussed in this dissertation, the factor that differentiates the two repeated measurements is the independent variable of interest. Research on how to statistically test mediation, moderation, and conditional process models in these designs has been minimal.