Meta-Analysis of Proportions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Contingency Tables Are Eaten by Large Birds of Prey
Case Study Case Study Example 9.3 beginning on page 213 of the text describes an experiment in which fish are placed in a large tank for a period of time and some Contingency Tables are eaten by large birds of prey. The fish are categorized by their level of parasitic infection, either uninfected, lightly infected, or highly infected. It is to the parasites' advantage to be in a fish that is eaten, as this provides Bret Hanlon and Bret Larget an opportunity to infect the bird in the parasites' next stage of life. The observed proportions of fish eaten are quite different among the categories. Department of Statistics University of Wisconsin|Madison Uninfected Lightly Infected Highly Infected Total October 4{6, 2011 Eaten 1 10 37 48 Not eaten 49 35 9 93 Total 50 45 46 141 The proportions of eaten fish are, respectively, 1=50 = 0:02, 10=45 = 0:222, and 37=46 = 0:804. Contingency Tables 1 / 56 Contingency Tables Case Study Infected Fish and Predation 2 / 56 Stacked Bar Graph Graphing Tabled Counts Eaten Not eaten 50 40 A stacked bar graph shows: I the sample sizes in each sample; and I the number of observations of each type within each sample. 30 This plot makes it easy to compare sample sizes among samples and 20 counts within samples, but the comparison of estimates of conditional Frequency probabilities among samples is less clear. 10 0 Uninfected Lightly Infected Highly Infected Contingency Tables Case Study Graphics 3 / 56 Contingency Tables Case Study Graphics 4 / 56 Mosaic Plot Mosaic Plot Eaten Not eaten 1.0 0.8 A mosaic plot replaces absolute frequencies (counts) with relative frequencies within each sample. -
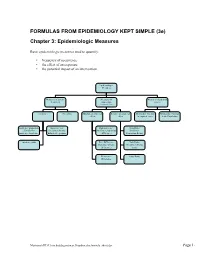
FORMULAS from EPIDEMIOLOGY KEPT SIMPLE (3E) Chapter 3: Epidemiologic Measures
FORMULAS FROM EPIDEMIOLOGY KEPT SIMPLE (3e) Chapter 3: Epidemiologic Measures Basic epidemiologic measures used to quantify: • frequency of occurrence • the effect of an exposure • the potential impact of an intervention. Epidemiologic Measures Measures of disease Measures of Measures of potential frequency association impact (“Measures of Effect”) Incidence Prevalence Absolute measures of Relative measures of Attributable Fraction Attributable Fraction effect effect in exposed cases in the Population Incidence proportion Incidence rate Risk difference Risk Ratio (Cumulative (incidence density, (Incidence proportion (Incidence Incidence, Incidence hazard rate, person- difference) Proportion Ratio) Risk) time rate) Incidence odds Rate Difference Rate Ratio (Incidence density (Incidence density difference) ratio) Prevalence Odds Ratio Difference Macintosh HD:Users:buddygerstman:Dropbox:eks:formula_sheet.doc Page 1 of 7 3.1 Measures of Disease Frequency No. of onsets Incidence Proportion = No. at risk at beginning of follow-up • Also called risk, average risk, and cumulative incidence. • Can be measured in cohorts (closed populations) only. • Requires follow-up of individuals. No. of onsets Incidence Rate = ∑person-time • Also called incidence density and average hazard. • When disease is rare (incidence proportion < 5%), incidence rate ≈ incidence proportion. • In cohorts (closed populations), it is best to sum individual person-time longitudinally. It can also be estimated as Σperson-time ≈ (average population size) × (duration of follow-up). Actuarial adjustments may be needed when the disease outcome is not rare. • In an open populations, Σperson-time ≈ (average population size) × (duration of follow-up). Examples of incidence rates in open populations include: births Crude birth rate (per m) = × m mid-year population size deaths Crude mortality rate (per m) = × m mid-year population size deaths < 1 year of age Infant mortality rate (per m) = × m live births No. -

Relative Risks, Odds Ratios and Cluster Randomised Trials . (1)
1 Relative Risks, Odds Ratios and Cluster Randomised Trials Michael J Campbell, Suzanne Mason, Jon Nicholl Abstract It is well known in cluster randomised trials with a binary outcome and a logistic link that the population average model and cluster specific model estimate different population parameters for the odds ratio. However, it is less well appreciated that for a log link, the population parameters are the same and a log link leads to a relative risk. This suggests that for a prospective cluster randomised trial the relative risk is easier to interpret. Commonly the odds ratio and the relative risk have similar values and are interpreted similarly. However, when the incidence of events is high they can differ quite markedly, and it is unclear which is the better parameter . We explore these issues in a cluster randomised trial, the Paramedic Practitioner Older People’s Support Trial3 . Key words: Cluster randomized trials, odds ratio, relative risk, population average models, random effects models 1 Introduction Given two groups, with probabilities of binary outcomes of π0 and π1 , the Odds Ratio (OR) of outcome in group 1 relative to group 0 is related to Relative Risk (RR) by: . If there are covariates in the model , one has to estimate π0 at some covariate combination. One can see from this relationship that if the relative risk is small, or the probability of outcome π0 is small then the odds ratio and the relative risk will be close. One can also show, using the delta method, that approximately (1). ________________________________________________________________________________ Michael J Campbell, Medical Statistics Group, ScHARR, Regent Court, 30 Regent St, Sheffield S1 4DA [email protected] 2 Note that this result is derived for non-clustered data. -

CHAPTER 3 Comparing Disease Frequencies
© Smartboy10/DigitalVision Vectors/Getty Images CHAPTER 3 Comparing Disease Frequencies LEARNING OBJECTIVES By the end of this chapter the reader will be able to: ■ Organize disease frequency data into a two-by-two table. ■ Describe and calculate absolute and relative measures of comparison, including rate/risk difference, population rate/risk difference, attributable proportion among the exposed and the total population and rate/risk ratio. ■ Verbally interpret each absolute and relative measure of comparison. ■ Describe the purpose of standardization and calculate directly standardized rates. ▸ Introduction Measures of disease frequency are the building blocks epidemiologists use to assess the effects of a disease on a population. Comparing measures of disease frequency organizes these building blocks in a meaningful way that allows one to describe the relationship between a characteristic and a disease and to assess the public health effect of the exposure. Disease frequencies can be compared between different populations or between subgroups within a population. For example, one might be interested in comparing disease frequencies between residents of France and the United States or between subgroups within the U.S. population according to demographic characteristics, such as race, gender, or socio- economic status; personal habits, such as alcoholic beverage consump- tion or cigarette smoking; and environmental factors, such as the level of air pollution. For example, one might compare incidence rates of coronary heart disease between residents of France and those of the United States or 57 58 Chapter 3 Comparing Disease Frequencies among U.S. men and women, Blacks and Whites, alcohol drinkers and nondrinkers, and areas with high and low pollution levels. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Outcome Reporting Bias in COVID-19 Mrna Vaccine Clinical Trials
medicina Perspective Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials Ronald B. Brown School of Public Health and Health Systems, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] Abstract: Relative risk reduction and absolute risk reduction measures in the evaluation of clinical trial data are poorly understood by health professionals and the public. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in Pfzier/BioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows: relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016; absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000. For the Moderna vaccine mRNA-1273, the appraisal shows: relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004; absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000. Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures. Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy. Keywords: mRNA vaccine; COVID-19 vaccine; vaccine efficacy; relative risk reduction; absolute risk reduction; number needed to vaccinate; outcome reporting bias; clinical epidemiology; critical appraisal; evidence-based medicine Citation: Brown, R.B. -

Copyright 2009, the Johns Hopkins University and John Mcgready. All Rights Reserved
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this site. Copyright 2009, The Johns Hopkins University and John McGready. All rights reserved. Use of these materials permitted only in accordance with license rights granted. Materials provided “AS IS”; no representations or warranties provided. User assumes all responsibility for use, and all liability related thereto, and must independently review all materials for accuracy and efficacy. May contain materials owned by others. User is responsible for obtaining permissions for use from third parties as needed. Section F Measures of Association: Risk Difference, Relative Risk, and the Odds Ratio Risk Difference Risk difference (attributable risk)—difference in proportions - Sample (estimated) risk difference - Example: the difference in risk of HIV for children born to HIV+ mothers taking AZT relative to HIV+ mothers taking placebo 3 Risk Difference From csi command, with 95% CI 4 Risk Difference Interpretation, sample estimate - If AZT was given to 1,000 HIV infected pregnant women, this would reduce the number of HIV positive infants by 150 (relative to the number of HIV positive infants born to 1,000 women not treated with AZT) Interpretation 95% CI - Study results suggest that the reduction in HIV positive births from 1,000 HIV positive pregnant women treated with AZT could range from 75 to 220 fewer than the number occurring if the -

Clinical Epidemiologic Definations
CLINICAL EPIDEMIOLOGIC DEFINATIONS 1. STUDY DESIGN Case-series: Report of a number of cases of disease. Cross-sectional study: Study design, concurrently measure outcome (disease) and risk factor. Compare proportion of diseased group with risk factor, with proportion of non-diseased group with risk factor. Case-control study: Retrospective comparison of exposures of persons with disease (cases) with those of persons without the disease (controls) (see Retrospective study). Retrospective study: Study design in which cases where individuals who had an outcome event in question are collected and analyzed after the outcomes have occurred (see also Case-control study). Cohort study: Follow-up of exposed and non-exposed defined groups, with a comparison of disease rates during the time covered. Prospective study: Study design where one or more groups (cohorts) of individuals who have not yet had the outcome event in question are monitored for the number of such events, which occur over time. Randomized controlled trial: Study design where treatments, interventions, or enrollment into different study groups are assigned by random allocation rather than by conscious decisions of clinicians or patients. If the sample size is large enough, this study design avoids problems of bias and confounding variables by assuring that both known and unknown determinants of outcome are evenly distributed between treatment and control groups. Bias (Syn: systematic error): Deviation of results or inferences from the truth, or processes leading to such deviation. See also Referral Bias, Selection Bias. Recall bias: Systematic error due to the differences in accuracy or completeness of recall to memory of past events or experiences. -

Meta-Analysis: Method for Quantitative Data Synthesis
Department of Health Sciences M.Sc. in Evidence Based Practice, M.Sc. in Health Services Research Meta-analysis: method for quantitative data synthesis Martin Bland Professor of Health Statistics University of York http://martinbland.co.uk/msc/ Adapted from work by Seokyung Hahn What is a meta-analysis? An optional component of a systematic review. A statistical technique for summarising the results of several studies into a single estimate. What does it do? identifies a common effect among a set of studies, allows an aggregated clearer picture to emerge, improves the precision of an estimate by making use of all available data. 1 When can you do a meta-analysis? When more than one study has estimated the effect of an intervention or of a risk factor, when there are no differences in participants, interventions and settings which are likely to affect outcome substantially, when the outcome in the different studies has been measured in similar ways, when the necessary data are available. A meta-analysis consists of three main parts: a pooled estimate and confidence interval for the treatment effect after combining all the studies, a test for whether the treatment or risk factor effect is statistically significant or not (i.e. does the effect differ from no effect more than would be expected by chance?), a test for heterogeneity of the effect on outcome between the included studies (i.e. does the effect vary across the studies more than would be expected by chance?). Example: migraine and ischaemic stroke BMJ 2005; 330: 63- . 2 Example: metoclopramide compared with placebo in reducing pain from acute migraine BMJ 2004; 329: 1369-72. -

Superiority by a Margin Tests for the Odds Ratio of Two Proportions
PASS Sample Size Software NCSS.com Chapter 197 Superiority by a Margin Tests for the Odds Ratio of Two Proportions Introduction This module provides power analysis and sample size calculation for superiority by a margin tests of the odds ratio in two-sample designs in which the outcome is binary. Users may choose between two popular test statistics commonly used for running the hypothesis test. The power calculations assume that independent, random samples are drawn from two populations. Example A superiority by a margin test example will set the stage for the discussion of the terminology that follows. Suppose that the current treatment for a disease works 70% of the time. A promising new treatment has been developed to the point where it can be tested. The researchers wish to show that the new treatment is better than the current treatment by at least some amount. In other words, does a clinically significant higher number of treated subjects respond to the new treatment? Clinicians want to demonstrate the new treatment is superior to the current treatment. They must determine, however, how much more effective the new treatment must be to be adopted. Should it be adopted if 71% respond? 72%? 75%? 80%? There is a percentage above 70% at which the difference between the two treatments is no longer considered ignorable. After thoughtful discussion with several clinicians, it was decided that if the odds ratio of new treatment to reference is at least 1.1, the new treatment would be adopted. This ratio is called the margin of superiority. The margin of superiority in this example is 1.1. -

As an "Odds Ratio", the Ratio of the Odds of Being Exposed by a Case Divided by the Odds of Being Exposed by a Control
STUDY DESIGNS IN BIOMEDICAL RESEARCH VALIDITY & SAMPLE SIZE Validity is an important concept; it involves the assessment against accepted absolute standards, or in a milder form, to see if the evaluation appears to cover its intended target or targets. INFERENCES & VALIDITIES Two major levels of inferences are involved in interpreting a study, a clinical trial The first level concerns Internal validity; the degree to which the investigator draws the correct conclusions about what actually happened in the study. The second level concerns External Validity (also referred to as generalizability or inference); the degree to which these conclusions could be appropriately applied to people and events outside the study. External Validity Internal Validity Truth in Truth in Findings in The Universe The Study The Study Research Question Study Plan Study Data A Simple Example: An experiment on the effect of Vitamin C on the prevention of colds could be simply conducted as follows. A number of n children (the sample size) are randomized; half were each give a 1,000-mg tablet of Vitamin C daily during the test period and form the “experimental group”. The remaining half , who made up the “control group” received “placebo” – an identical tablet containing no Vitamin C – also on a daily basis. At the end, the “Number of colds per child” could be chosen as the outcome/response variable, and the means of the two groups are compared. Assignment of the treatments (factor levels: Vitamin C or Placebo) to the experimental units (children) was performed using a process called “randomization”. The purpose of randomization was to “balance” the characteristics of the children in each of the treatment groups, so that the difference in the response variable, the number of cold episodes per child, can be rightly attributed to the effect of the predictor – the difference between Vitamin C and Placebo.