Caveant Omnes David H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Meta-Analysis of Proportions
NCSS Statistical Software NCSS.com Chapter 456 Meta-Analysis of Proportions Introduction This module performs a meta-analysis of a set of two-group, binary-event studies. These studies have a treatment group (arm) and a control group. The results of each study may be summarized as counts in a 2-by-2 table. The program provides a complete set of numeric reports and plots to allow the investigation and presentation of the studies. The plots include the forest plot, radial plot, and L’Abbe plot. Both fixed-, and random-, effects models are available for analysis. Meta-Analysis refers to methods for the systematic review of a set of individual studies with the aim to combine their results. Meta-analysis has become popular for a number of reasons: 1. The adoption of evidence-based medicine which requires that all reliable information is considered. 2. The desire to avoid narrative reviews which are often misleading. 3. The desire to interpret the large number of studies that may have been conducted about a specific treatment. 4. The desire to increase the statistical power of the results be combining many small-size studies. The goals of meta-analysis may be summarized as follows. A meta-analysis seeks to systematically review all pertinent evidence, provide quantitative summaries, integrate results across studies, and provide an overall interpretation of these studies. We have found many books and articles on meta-analysis. In this chapter, we briefly summarize the information in Sutton et al (2000) and Thompson (1998). Refer to those sources for more details about how to conduct a meta- analysis. -

Teacher Guide 12.1 Gambling Page 1
TEACHER GUIDE 12.1 GAMBLING PAGE 1 Standard 12: The student will explain and evaluate the financial impact and consequences of gambling. Risky Business Priority Academic Student Skills Personal Financial Literacy Objective 12.1: Analyze the probabilities involved in winning at games of chance. Objective 12.2: Evaluate costs and benefits of gambling to Simone, Paula, and individuals and society (e.g., family budget; addictive behaviors; Randy meet in the library and the local and state economy). every afternoon to work on their homework. Here is what is going on. Ryan is flipping a coin, and he is not cheating. He has just flipped seven heads in a row. Is Ryan’s next flip more likely to be: Heads; Tails; or Heads and tails are equally likely. Paula says heads because the first seven were heads, so the next one will probably be heads too. Randy says tails. The first seven were heads, so the next one must be tails. Lesson Objectives Simone says that it could be either heads or tails Recognize gambling as a form of risk. because both are equally Calculate the probabilities of winning in games of chance. likely. Who is correct? Explore the potential benefits of gambling for society. Explore the potential costs of gambling for society. Evaluate the personal costs and benefits of gambling. © 2008. Oklahoma State Department of Education. All rights reserved. Teacher Guide 12.1 2 Personal Financial Literacy Vocabulary Dependent event: The outcome of one event affects the outcome of another, changing the TEACHING TIP probability of the second event. This lesson is based on risk. -

Contingency Tables Are Eaten by Large Birds of Prey
Case Study Case Study Example 9.3 beginning on page 213 of the text describes an experiment in which fish are placed in a large tank for a period of time and some Contingency Tables are eaten by large birds of prey. The fish are categorized by their level of parasitic infection, either uninfected, lightly infected, or highly infected. It is to the parasites' advantage to be in a fish that is eaten, as this provides Bret Hanlon and Bret Larget an opportunity to infect the bird in the parasites' next stage of life. The observed proportions of fish eaten are quite different among the categories. Department of Statistics University of Wisconsin|Madison Uninfected Lightly Infected Highly Infected Total October 4{6, 2011 Eaten 1 10 37 48 Not eaten 49 35 9 93 Total 50 45 46 141 The proportions of eaten fish are, respectively, 1=50 = 0:02, 10=45 = 0:222, and 37=46 = 0:804. Contingency Tables 1 / 56 Contingency Tables Case Study Infected Fish and Predation 2 / 56 Stacked Bar Graph Graphing Tabled Counts Eaten Not eaten 50 40 A stacked bar graph shows: I the sample sizes in each sample; and I the number of observations of each type within each sample. 30 This plot makes it easy to compare sample sizes among samples and 20 counts within samples, but the comparison of estimates of conditional Frequency probabilities among samples is less clear. 10 0 Uninfected Lightly Infected Highly Infected Contingency Tables Case Study Graphics 3 / 56 Contingency Tables Case Study Graphics 4 / 56 Mosaic Plot Mosaic Plot Eaten Not eaten 1.0 0.8 A mosaic plot replaces absolute frequencies (counts) with relative frequencies within each sample. -

Relative Risks, Odds Ratios and Cluster Randomised Trials . (1)
1 Relative Risks, Odds Ratios and Cluster Randomised Trials Michael J Campbell, Suzanne Mason, Jon Nicholl Abstract It is well known in cluster randomised trials with a binary outcome and a logistic link that the population average model and cluster specific model estimate different population parameters for the odds ratio. However, it is less well appreciated that for a log link, the population parameters are the same and a log link leads to a relative risk. This suggests that for a prospective cluster randomised trial the relative risk is easier to interpret. Commonly the odds ratio and the relative risk have similar values and are interpreted similarly. However, when the incidence of events is high they can differ quite markedly, and it is unclear which is the better parameter . We explore these issues in a cluster randomised trial, the Paramedic Practitioner Older People’s Support Trial3 . Key words: Cluster randomized trials, odds ratio, relative risk, population average models, random effects models 1 Introduction Given two groups, with probabilities of binary outcomes of π0 and π1 , the Odds Ratio (OR) of outcome in group 1 relative to group 0 is related to Relative Risk (RR) by: . If there are covariates in the model , one has to estimate π0 at some covariate combination. One can see from this relationship that if the relative risk is small, or the probability of outcome π0 is small then the odds ratio and the relative risk will be close. One can also show, using the delta method, that approximately (1). ________________________________________________________________________________ Michael J Campbell, Medical Statistics Group, ScHARR, Regent Court, 30 Regent St, Sheffield S1 4DA [email protected] 2 Note that this result is derived for non-clustered data. -

Odds: Gambling, Law and Strategy in the European Union Anastasios Kaburakis* and Ryan M Rodenberg†
63 Odds: Gambling, Law and Strategy in the European Union Anastasios Kaburakis* and Ryan M Rodenberg† Contemporary business law contributions argue that legal knowledge or ‘legal astuteness’ can lead to a sustainable competitive advantage.1 Past theses and treatises have led more academic research to endeavour the confluence between law and strategy.2 European scholars have engaged in the Proactive Law Movement, recently adopted and incorporated into policy by the European Commission.3 As such, the ‘many futures of legal strategy’ provide * Dr Anastasios Kaburakis is an assistant professor at the John Cook School of Business, Saint Louis University, teaching courses in strategic management, sports business and international comparative gaming law. He holds MS and PhD degrees from Indiana University-Bloomington and a law degree from Aristotle University in Thessaloniki, Greece. Prior to academia, he practised law in Greece and coached basketball at the professional club and national team level. He currently consults international sport federations, as well as gaming operators on regulatory matters and policy compliance strategies. † Dr Ryan Rodenberg is an assistant professor at Florida State University where he teaches sports law analytics. He earned his JD from the University of Washington-Seattle and PhD from Indiana University-Bloomington. Prior to academia, he worked at Octagon, Nike and the ATP World Tour. 1 See C Bagley, ‘What’s Law Got to Do With It?: Integrating Law and Strategy’ (2010) 47 American Business Law Journal 587; C -
3. Probability Theory
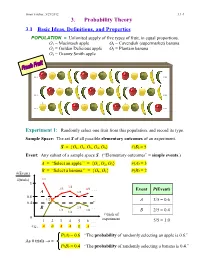
Ismor Fischer, 5/29/2012 3.1-1 3. Probability Theory 3.1 Basic Ideas, Definitions, and Properties POPULATION = Unlimited supply of five types of fruit, in equal proportions. O1 = Macintosh apple O4 = Cavendish (supermarket) banana O2 = Golden Delicious apple O5 = Plantain banana O3 = Granny Smith apple … … … … … … Experiment 1: Randomly select one fruit from this population, and record its type. Sample Space: The set S of all possible elementary outcomes of an experiment. S = {O1, O2, O3, O4, O5} #(S) = 5 Event: Any subset of a sample space S. (“Elementary outcomes” = simple events.) A = “Select an apple.” = {O1, O2, O3} #(A) = 3 B = “Select a banana.” = {O , O } #(B) = 2 #(Event) 4 5 #(trials) 1/1 1 3/4 2/3 4/6 . Event P(Event) A 3/5 0.6 1/2 A 3/5 = 0.6 0.4 B 2/5 . 1/3 2/6 1/4 B 2/5 = 0.4 # trials of 0 experiment 1 2 3 4 5 6 . 5/5 = 1.0 e.g., A B A A B A . P(A) = 0.6 “The probability of randomly selecting an apple is 0.6.” As # trials → ∞ P(B) = 0.4 “The probability of randomly selecting a banana is 0.4.” Ismor Fischer, 5/29/2012 3.1-2 General formulation may be facilitated with the use of a Venn diagram: Experiment ⇒ Sample Space: S = {O1, O2, …, Ok} #(S) = k A Om+1 Om+2 O2 O1 O3 Om+3 O 4 . Om . Ok Event A = {O1, O2, …, Om} ⊆ S #(A) = m ≤ k Definition: The probability of event A, denoted P(A), is the long-run relative frequency with which A is expected to occur, as the experiment is repeated indefinitely. -

Probability Cheatsheet V2.0 Thinking Conditionally Law of Total Probability (LOTP)
Probability Cheatsheet v2.0 Thinking Conditionally Law of Total Probability (LOTP) Let B1;B2;B3; :::Bn be a partition of the sample space (i.e., they are Compiled by William Chen (http://wzchen.com) and Joe Blitzstein, Independence disjoint and their union is the entire sample space). with contributions from Sebastian Chiu, Yuan Jiang, Yuqi Hou, and Independent Events A and B are independent if knowing whether P (A) = P (AjB )P (B ) + P (AjB )P (B ) + ··· + P (AjB )P (B ) Jessy Hwang. Material based on Joe Blitzstein's (@stat110) lectures 1 1 2 2 n n A occurred gives no information about whether B occurred. More (http://stat110.net) and Blitzstein/Hwang's Introduction to P (A) = P (A \ B1) + P (A \ B2) + ··· + P (A \ Bn) formally, A and B (which have nonzero probability) are independent if Probability textbook (http://bit.ly/introprobability). Licensed and only if one of the following equivalent statements holds: For LOTP with extra conditioning, just add in another event C! under CC BY-NC-SA 4.0. Please share comments, suggestions, and errors at http://github.com/wzchen/probability_cheatsheet. P (A \ B) = P (A)P (B) P (AjC) = P (AjB1;C)P (B1jC) + ··· + P (AjBn;C)P (BnjC) P (AjB) = P (A) P (AjC) = P (A \ B1jC) + P (A \ B2jC) + ··· + P (A \ BnjC) P (BjA) = P (B) Last Updated September 4, 2015 Special case of LOTP with B and Bc as partition: Conditional Independence A and B are conditionally independent P (A) = P (AjB)P (B) + P (AjBc)P (Bc) given C if P (A \ BjC) = P (AjC)P (BjC). -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Meta-Analysis: Method for Quantitative Data Synthesis
Department of Health Sciences M.Sc. in Evidence Based Practice, M.Sc. in Health Services Research Meta-analysis: method for quantitative data synthesis Martin Bland Professor of Health Statistics University of York http://martinbland.co.uk/msc/ Adapted from work by Seokyung Hahn What is a meta-analysis? An optional component of a systematic review. A statistical technique for summarising the results of several studies into a single estimate. What does it do? identifies a common effect among a set of studies, allows an aggregated clearer picture to emerge, improves the precision of an estimate by making use of all available data. 1 When can you do a meta-analysis? When more than one study has estimated the effect of an intervention or of a risk factor, when there are no differences in participants, interventions and settings which are likely to affect outcome substantially, when the outcome in the different studies has been measured in similar ways, when the necessary data are available. A meta-analysis consists of three main parts: a pooled estimate and confidence interval for the treatment effect after combining all the studies, a test for whether the treatment or risk factor effect is statistically significant or not (i.e. does the effect differ from no effect more than would be expected by chance?), a test for heterogeneity of the effect on outcome between the included studies (i.e. does the effect vary across the studies more than would be expected by chance?). Example: migraine and ischaemic stroke BMJ 2005; 330: 63- . 2 Example: metoclopramide compared with placebo in reducing pain from acute migraine BMJ 2004; 329: 1369-72. -

Superiority by a Margin Tests for the Odds Ratio of Two Proportions
PASS Sample Size Software NCSS.com Chapter 197 Superiority by a Margin Tests for the Odds Ratio of Two Proportions Introduction This module provides power analysis and sample size calculation for superiority by a margin tests of the odds ratio in two-sample designs in which the outcome is binary. Users may choose between two popular test statistics commonly used for running the hypothesis test. The power calculations assume that independent, random samples are drawn from two populations. Example A superiority by a margin test example will set the stage for the discussion of the terminology that follows. Suppose that the current treatment for a disease works 70% of the time. A promising new treatment has been developed to the point where it can be tested. The researchers wish to show that the new treatment is better than the current treatment by at least some amount. In other words, does a clinically significant higher number of treated subjects respond to the new treatment? Clinicians want to demonstrate the new treatment is superior to the current treatment. They must determine, however, how much more effective the new treatment must be to be adopted. Should it be adopted if 71% respond? 72%? 75%? 80%? There is a percentage above 70% at which the difference between the two treatments is no longer considered ignorable. After thoughtful discussion with several clinicians, it was decided that if the odds ratio of new treatment to reference is at least 1.1, the new treatment would be adopted. This ratio is called the margin of superiority. The margin of superiority in this example is 1.1. -

As an "Odds Ratio", the Ratio of the Odds of Being Exposed by a Case Divided by the Odds of Being Exposed by a Control
STUDY DESIGNS IN BIOMEDICAL RESEARCH VALIDITY & SAMPLE SIZE Validity is an important concept; it involves the assessment against accepted absolute standards, or in a milder form, to see if the evaluation appears to cover its intended target or targets. INFERENCES & VALIDITIES Two major levels of inferences are involved in interpreting a study, a clinical trial The first level concerns Internal validity; the degree to which the investigator draws the correct conclusions about what actually happened in the study. The second level concerns External Validity (also referred to as generalizability or inference); the degree to which these conclusions could be appropriately applied to people and events outside the study. External Validity Internal Validity Truth in Truth in Findings in The Universe The Study The Study Research Question Study Plan Study Data A Simple Example: An experiment on the effect of Vitamin C on the prevention of colds could be simply conducted as follows. A number of n children (the sample size) are randomized; half were each give a 1,000-mg tablet of Vitamin C daily during the test period and form the “experimental group”. The remaining half , who made up the “control group” received “placebo” – an identical tablet containing no Vitamin C – also on a daily basis. At the end, the “Number of colds per child” could be chosen as the outcome/response variable, and the means of the two groups are compared. Assignment of the treatments (factor levels: Vitamin C or Placebo) to the experimental units (children) was performed using a process called “randomization”. The purpose of randomization was to “balance” the characteristics of the children in each of the treatment groups, so that the difference in the response variable, the number of cold episodes per child, can be rightly attributed to the effect of the predictor – the difference between Vitamin C and Placebo.