Descriptive and Analytic Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
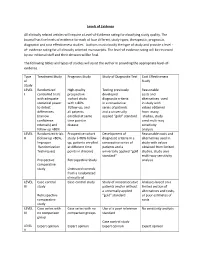
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

TUTORIAL in BIOSTATISTICS: the Self-Controlled Case Series Method
STATISTICS IN MEDICINE Statist. Med. 2005; 0:1–31 Prepared using simauth.cls [Version: 2002/09/18 v1.11] TUTORIAL IN BIOSTATISTICS: The self-controlled case series method Heather J. Whitaker1, C. Paddy Farrington1, Bart Spiessens2 and Patrick Musonda1 1 Department of Statistics, The Open University, Milton Keynes, MK7 6AA, UK. 2 GlaxoSmithKline Biologicals, Rue de l’Institut 89, B-1330 Rixensart, Belgium. SUMMARY The self-controlled case series method was developed to investigate associations between acute outcomes and transient exposures, using only data on cases, that is, on individuals who have experienced the outcome of interest. Inference is within individuals, and hence fixed covariates effects are implicitly controlled for within a proportional incidence framework. We describe the origins, assumptions, limitations, and uses of the method. The rationale for the model and the derivation of the likelihood are explained in detail using a worked example on vaccine safety. Code for fitting the model in the statistical package STATA is described. Two further vaccine safety data sets are used to illustrate a range of modelling issues and extensions of the basic model. Some brief pointers on the design of case series studies are provided. The data sets, STATA code, and further implementation details in SAS, GENSTAT and GLIM are available from an associated website. key words: case series; conditional likelihood; control; epidemiology; modelling; proportional incidence Copyright c 2005 John Wiley & Sons, Ltd. 1. Introduction The self-controlled case series method, or case series method for short, provides an alternative to more established cohort or case-control methods for investigating the association between a time-varying exposure and an outcome event. -

Supplementary File
Supplementary file Table S1. Characteristics of studies included in the systematic review. Authors, reference Type of article Number of patients Type of stroke Valderrama [1] case report 1 AIS Oxley [2] case series 5 AIS Morassi [3] case series 6 AIS (n = 4), HS (n = 2) Tunc [4] case series 4 AIS Avula [5] case series 4 AIS (n = 3), TIA (n = 1) Oliver [6] case report 1 AIS Beyrouti [7] case series 6 AIS Al Saiegh [8] case series 2 AIS (n = 1), SAH (n = 1) Gunasekaran [9] case report 1 AIS Goldberg [10] case report 1 AIS Viguier [11] case report 1 AIS Escalard [12] case series/ case control 10 AIS Wang [13] case series 5 AIS Fara [14] case series 3 AIS Moshayedi [15] case report 1 AIS Deliwala [16] case report 1 AIS Sharafi-Razavi [17] case report 1 HS retrospective cohort study (case Jain [18] 35 AIS (n = 26) HS (n = 9) control) retrospective cohort study (case Yaghi [19] 32 AIS control) AIS (n = 35), TIA (n = 5), HS Benussi [20] retrospective cohort study 43 (n = 3) Rudilosso [21] case report 1 AIS Lodigiani [22] retrospective cohort study 9 AIS Malentacchi [23] case report 1 AIS J. Clin. Med. 2020, 9, x; doi: FOR PEER REVIEW www.mdpi.com/journal/jcm J. Clin. Med. 2020, 9, x FOR PEER REVIEW 2 of 8 retrospective cohort study/case Scullen [24] 2 HS + AIS (n = 1), AIS (n = 1) series retrospective cohort study/ case AIS (n = 17), ST (n = 2), HS Sweid [25] 22 series (n = 3) AIS – acute ischemic stroke; HS – haemorrhagic stroke; SAH – subarachnoid haemorrhage; ST – sinus thrombosis; TIA-transient ischemic attack. -

The Very Process of Taking a Pill May Create Expectations in a Patient Which May Affect Reactions to the Pill
Ch 1 Pause for Thought Questions p. 36 1- Why is it important to use placebos and a double-blind approach in some studies? The very process of taking a pill may create expectations in a patient which may affect reactions to the pill. To help eliminate the role of patient expectation during testing, a placebo (or fake medicine) is used on subjects in a control group. A researcher’s expectations may also affect results. Therefore, double-blind studies are often used. In such studies, neither the researcher nor the participants know which group is which. 2- Assume that researchers find that people’s memories are sharpest right after they’ve eaten lunch. What hidden variables may have affected these results? Other variables that may play a role (hidden variables): Time of Day Type of food eaten Meaning of “lunch” - May mean “time away from work” - Subjective State of feeling free (not food) helps memory. Socializing at lunch may make a person more alert and then able to take-in more info and retain it longer. 3- How might you use the scientific method to study factors that affect obedience? Devise a simple study, and identify the following: hypothesis, subjects, independent variable, dependent variable, experimental group, and control group. Hypothesis: If employee fears/believes they will be punished if they don’t follow directives, then they will be obedient to directives of bosses/people in “higher” positions than themselves in the workplace. Subjects: Employees Independent Variable: Directive given with harsh consequences for not following through. Dependent Variable: Obedience (Level of) Experimental Group: Group with “harsh” bosses (very by the book; give extreme consequences) present during their work day. -

Journal of Clinical Trails Market Analysis Advances in Clinical Research and Clinical Trials 2020
Journal of Clinical Trails Market Analysis Advances in Clinical Research and Clinical Trials 2020 Raymond Chong The research report on Global Clinical Trials Market 2019 Other prominent players in the value chain include Mednet keenly analyzes significant features of the industry. The Solutions, Arisglobal, eClinForce Inc., DZS Software Solutions, DSG, analysis servers market size, latest trends, drivers, threats, Inc., Guger Technologies Inc., ICON, Plc., ChemWare Inc., and opportunities, as well as key market segments. It is based on iWeb Technologies Limited. past data and present market needs. Also, involve distinct business approaches accepted by the decision makers. Those intensify growth and make a remarkable stand in the industry. The Clinical Trials market will grow with a significant CAGR between 2019 to 2028. The report segregates the complete market on the basis of key players, geographical areas, and segments. Increasing demand for new medical equipments and medicines among end users, coupled with growing investment for research and development activities for development of effective medicines are major factors driving growth of the global clinical trials market. In addition, increasing number of individuals suffering from chronic diseases as well as changing The clinical trials market is projected to arrive at USD 1.76 conditions and nature of certain types of chronic diseases is billion by 2025, from USD 1.04 billion in 2017 growing at a another factor anticipated to support growth of the global CAGR of 6.7 percent from 2018 to 2025. An upcoming clinical trials market to significant extent. market report contains data for historic year 2016, and the base year of calculation is 2017. -

Chronic Lymphocytic Leukemia in a Black
L OPEN ACCESS Freely available online al of euk rn em u i o a J Journal of Leukemia ISSN: 2329-6917 Case Report Chronic Lymphocytic Leukemia in A Black African Man: A Cameroonian Case Report Raspail Carrel Founou1*, Julius Nwobegahay2, Regine Gandji3, Cedrice Tsayem4, Sandra Yopa5, Martin Kuete6 and Luria Leslie Founou7 1Department of Clinical Microbiology, Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM), Yaounde, Cameroon; 2Military Health Research Centre (CRESAR), Yaounde, Cameroon; 3Department of Biological Sciences, Higher Institute of Medical Technology, Yaoundé, Cameroon; 4Department of Clinical Biochemistry, Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM), Yaounde, Cameroon; 5Department of Emergency, District Hospital of Biyem-Assi, Yaounde, Cameroon; 6Department of Biomedical and Applied Health, Faculty of Health Sciences, Université des Montagnes, Bangante, Cameroon; 7Department of Food Safety and Environmental Microbiology, Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM), Yaounde, Cameroo ABSTRACT Chronic Lymphocytic Leukemia (CLL) is an acquired monoclonal disorder characterized by a gradual accumulation of functionally incompetent lymphocytes. It generally presents a clonal B cells arrested in the B-cell differentiation pathway that resemble morphologically to mature lymphocytes in the peripheral blood. There is a scarcity of CLL data among sub-Saharan African countries such as Cameroon. We herein report a case of CLL that remained stable over a period of seven years in a 54 years old Black African man. The patient had no history of exposure to toxic chemicals or ionizing radiation and presented with several complaints and clinical symptoms. Clinical and laboratory investigations indicated a CLL in stage B of the Binet staging system. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Case Series: COVID-19 Infection Causing New-Onset Diabetes Mellitus?
Endocrinology & Metabolism International Journal Case Series Open Access Case series: COVID-19 infection causing new-onset diabetes mellitus? Abstract Volume 9 Issue 1 - 2021 Objectives: This paper seeks to explore the hypothesis of the potential diabetogenic effect 1 2 of SARS-COV-2 (Severe Acute respiratory syndrome coronavirus). Rujuta Katkar, Narasa Raju Madam 1Consultant Endocrinologist at Yuma Regional Medical Center, Case series presentation: We present a case series of observation among 8 patients of age USA 2 group ranging from 34 to 74 years with a BMI range of 26.61 to 53.21 Kilogram/square Hospitalist at Yuma Regional Medical Center, USA meters that developed new-onset diabetes after COVID-19 infection. Correspondence: Rujuta Katkar, MD, Consultant Severe Acute Respiratory Syndrome Coronavirus (SARS-COV-2), commonly known as Endocrinologist at Yuma Regional Medical Center, 2851 S Coronavirus or COVID-19(Coronavirus infectious disease), gains entry into the cells by Avenue B, Bldg.20, Yuma, AZ-85364, USA, Tel 2034359976, binding to the Angiotensin-converting enzyme-2(ACE-2) receptors located in essential Email metabolic tissues including the pancreas, adipose tissue, small intestine, and kidneys. Received: March 25, 2021 | Published: April 02, 2021 The evidence reviewed from the scientific literature describes how ACE 2 receptors play a role in the pathogenesis of diabetes and the plausible interaction of SARS-COV-2 with ACE 2 receptors in metabolic organs and tissues. Conclusion: The 8 patients without a past medical history of diabetes admitted with COVID-19 infection developed new-onset diabetes mellitus due to plausible interaction of SARS-COV-2 with ACE 2 receptors. -

Impact of Demographic Factors on Impulse Buying Behavior of Consumers in Multan-Pakistan
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals European Journal of Business and Management www.iiste.org ISSN 2222-1905 (Paper) ISSN 2222-2839 (Online) Vol.7, No.22, 2015 Impact of Demographic Factors on Impulse Buying Behavior of Consumers in Multan-Pakistan Prof. Dr. Abdul Ghafoor Awan Dean, Faculty of Management and Social Sciences, Institute of Southern Punjab, Multan Nayyar Abbas MS Scholar, Department of Business Administration, Institute of Southern Punjab, Multan Abstract The purpose of this study is to investigate the effect of demographic factors (gender, age, income, and education) on impulse buying behavior of consumers in Multan. The study adopted quantitative approach. A structured questionnaire was used to survey 250 respondents (104 males and 146 females) who were selected using non- probability convenience sampling. Data were collected in different educational institutions of Multan. Different statistical methods like multiple regression, chi-square test and simple descriptive techniques were used to derive results from the data collected with the help of SPSS 17.0. The multiple regression and chi-square test results revealed that gender and age were significantly and inversely associated with impulse buying behavior of consumers. The results further indicated that income and education were significantly and directly associated with impulse buying behavior of consumers. The ANOVA results indicated that consumers’ demographic characteristic had significant influence on impulse buying and demographic characteristics (gender, age, income, and education) affect simultaneously impulse buying behavior of consumers. The findings of the study were consistent and supported by previous studies. -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Using a Study on Arsenic Ingestion and Bladder Cancer As an Example How-Ran Guo1,2,3
Guo BMC Public Health 2011, 11:820 http://www.biomedcentral.com/1471-2458/11/820 RESEARCHARTICLE Open Access Age adjustment in ecological studies: using a study on arsenic ingestion and bladder cancer as an example How-Ran Guo1,2,3 Abstract Background: Despite its limitations, ecological study design is widely applied in epidemiology. In most cases, adjustment for age is necessary, but different methods may lead to different conclusions. To compare three methods of age adjustment, a study on the associations between arsenic in drinking water and incidence of bladder cancer in 243 townships in Taiwan was used as an example. Methods: A total of 3068 cases of bladder cancer, including 2276 men and 792 women, were identified during a ten-year study period in the study townships. Three methods were applied to analyze the same data set on the ten-year study period. The first (Direct Method) applied direct standardization to obtain standardized incidence rate and then used it as the dependent variable in the regression analysis. The second (Indirect Method) applied indirect standardization to obtain standardized incidence ratio and then used it as the dependent variable in the regression analysis instead. The third (Variable Method) used proportions of residents in different age groups as a part of the independent variables in the multiple regression models. Results: All three methods showed a statistically significant positive association between arsenic exposure above 0.64 mg/L and incidence of bladder cancer in men and women, but different results were observed for the other exposure categories. In addition, the risk estimates obtained by different methods for the same exposure category were all different. -
Sensitivity and Specificity. Wikipedia. Last Modified on 16 November 2013
Sensitivity and specificity - Wikipedia, the free encyclopedia Create account Log in Article Talk Read Edit View Sensitivity and specificity From Wikipedia, the free encyclopedia Main page Contents Sensitivity and specificity are statistical measures of the performance of a binary classification test, also known in statistics as Featured content classification function. Sensitivity (also called the true positive rate, or the recall rate in some fields) measures the proportion of Current events actual positives which are correctly identified as such (e.g. the percentage of sick people who are correctly identified as having Random article the condition). Specificity measures the proportion of negatives which are correctly identified as such (e.g. the percentage of Donate to Wikipedia healthy people who are correctly identified as not having the condition, sometimes called the true negative rate). These two measures are closely related to the concepts of type I and type II errors. A perfect predictor would be described as 100% Interaction sensitive (i.e. predicting all people from the sick group as sick) and 100% specific (i.e. not predicting anyone from the healthy Help group as sick); however, theoretically any predictor will possess a minimum error bound known as the Bayes error rate. About Wikipedia For any test, there is usually a trade-off between the measures. For example: in an airport security setting in which one is testing Community portal for potential threats to safety, scanners may be set to trigger on low-risk items like belt buckles and keys (low specificity), in Recent changes order to reduce the risk of missing objects that do pose a threat to the aircraft and those aboard (high sensitivity).