Cohort Studies and Relative Risks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Challenging Issues in Clinical Trial Design: Part 4 of a 4-Part Series on Statistics for Clinical Trials
Challenging Issues in Clinical Trial Design: Part 4 of a 4-part Series on Statistics for Clinical Trials Brief title: Challenges in Trial Design Stuart J. Pocock, PHD,* Tim C. Clayton, MSC,* Gregg W. Stone, MD† From the: *London School of Hygiene and Tropical Medicine, London, United Kingdom; †Columbia University Medical Center, New York-Presbyterian Hospital and the Cardiovascular Research Foundation, New York, New York <COR> Reprint requests and correspondence: Prof. Stuart J. Pocock, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, Keppel Street, London, WC1E 7HT, United Kingdom Telephone: +44 20 7927 2413 Fax: +44 20 7637 2853 E-mail: [email protected] Disclosures: The authors declare no conflicts of interest for this paper. 1 Abstract As a sequel to last week’s article on the fundamentals of clinical trial design, this article tackles related controversial issues: noninferiority trials; the value of factorial designs; the importance and challenges of strategy trials; Data Monitoring Committees (including when to stop a trial early); and the role of adaptive designs. All topics are illustrated by relevant examples from cardiology trials. <KW>Key words: Noninferiority trials; Factorial designs; Strategy trials, Data Monitoring Committees; Statistical stopping guidelines; Adaptive designs; Randomized Controlled Trials As Topic; Abbreviations ACS = acute coronary syndrome CABG = coronary artery bypass graft CI = confidence interval CV = cardiovascular DMC = Data Monitoring Committee FDA = Food and Drug Administration MACE = major adverse cardiovascular event OMT = optimal medical therapy PCI = percutaneous coronary intervention 2 Introduction Randomized controlled trials are the cornerstone of clinical guidelines informing best therapeutic practices, however their design and interpretation may be complex and nuanced. -
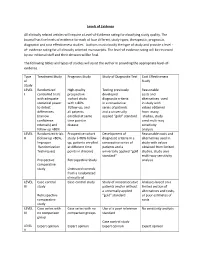
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

Limitations of the Odds Ratio in Gauging the Performance of a Diagnostic, Prognostic, Or Screening Marker
American Journal of Epidemiology Vol. 159, No. 9 Copyright © 2004 by the Johns Hopkins Bloomberg School of Public Health Printed in U.S.A. All rights reserved DOI: 10.1093/aje/kwh101 Limitations of the Odds Ratio in Gauging the Performance of a Diagnostic, Prognostic, or Screening Marker Margaret Sullivan Pepe1,2, Holly Janes2, Gary Longton1, Wendy Leisenring1,2,3, and Polly Newcomb1 1 Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA. 2 Department of Biostatistics, University of Washington, Seattle, WA. 3 Division of Clinical Research, Fred Hutchinson Cancer Research Center, Seattle, WA. Downloaded from Received for publication June 24, 2003; accepted for publication October 28, 2003. A marker strongly associated with outcome (or disease) is often assumed to be effective for classifying persons http://aje.oxfordjournals.org/ according to their current or future outcome. However, for this assumption to be true, the associated odds ratio must be of a magnitude rarely seen in epidemiologic studies. In this paper, an illustration of the relation between odds ratios and receiver operating characteristic curves shows, for example, that a marker with an odds ratio of as high as 3 is in fact a very poor classification tool. If a marker identifies 10% of controls as positive (false positives) and has an odds ratio of 3, then it will correctly identify only 25% of cases as positive (true positives). The authors illustrate that a single measure of association such as an odds ratio does not meaningfully describe a marker’s ability to classify subjects. Appropriate statistical methods for assessing and reporting the classification power of a marker are described. -

Guidance for Industry E2E Pharmacovigilance Planning
Guidance for Industry E2E Pharmacovigilance Planning U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2005 ICH Guidance for Industry E2E Pharmacovigilance Planning Additional copies are available from: Office of Training and Communication Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane Rockville, MD 20857 (Tel) 301-827-4573 http://www.fda.gov/cder/guidance/index.htm Office of Communication, Training and Manufacturers Assistance, HFM-40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike, Rockville, MD 20852-1448 http://www.fda.gov/cber/guidelines.htm. (Tel) Voice Information System at 800-835-4709 or 301-827-1800 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2005 ICH Contains Nonbinding Recommendations TABLE OF CONTENTS I. INTRODUCTION (1, 1.1) ................................................................................................... 1 A. Background (1.2) ..................................................................................................................2 B. Scope of the Guidance (1.3)...................................................................................................2 II. SAFETY SPECIFICATION (2) ..................................................................................... -

Survival Analysis: Part I — Analysis Korean Journal of Anesthesiology of Time-To-Event
Statistical Round pISSN 2005-6419 • eISSN 2005-7563 KJA Survival analysis: Part I — analysis Korean Journal of Anesthesiology of time-to-event Junyong In1 and Dong Kyu Lee2 Department of Anesthesiology and Pain Medicine, 1Dongguk University Ilsan Hospital, Goyang, 2Guro Hospital, Korea University School of Medicine, Seoul, Korea Length of time is a variable often encountered during data analysis. Survival analysis provides simple, intuitive results concerning time-to-event for events of interest, which are not confined to death. This review introduces methods of ana- lyzing time-to-event. The Kaplan-Meier survival analysis, log-rank test, and Cox proportional hazards regression model- ing method are described with examples of hypothetical data. Keywords: Censored data; Cox regression; Hazard ratio; Kaplan-Meier method; Log-rank test; Medical statistics; Power analysis; Proportional hazards; Sample size; Survival analysis. Introduction mation of the time [2]. The Korean Journal of Anesthesiology has thus far published In a clinical trial or clinical study, an investigational treat- several papers using survival analysis for clinical outcomes: a ment is administered to subjects, and the resulting outcome data comparison of 90-day survival rate for pressure ulcer develop- are collected and analyzed after a certain period of time. Most ment or non-development after surgery under general anesthesia statistical analysis methods do not include the length of time [3], a comparison of postoperative 5-year recurrence rate in as a variable, and analysis is made only on the trial outcomes patients with breast cancer depending on the type of anesthetic upon completion of the study period, as specified in the study agent used [4], and a comparison of airway intubation success protocol. -

Relative Risks, Odds Ratios and Cluster Randomised Trials . (1)
1 Relative Risks, Odds Ratios and Cluster Randomised Trials Michael J Campbell, Suzanne Mason, Jon Nicholl Abstract It is well known in cluster randomised trials with a binary outcome and a logistic link that the population average model and cluster specific model estimate different population parameters for the odds ratio. However, it is less well appreciated that for a log link, the population parameters are the same and a log link leads to a relative risk. This suggests that for a prospective cluster randomised trial the relative risk is easier to interpret. Commonly the odds ratio and the relative risk have similar values and are interpreted similarly. However, when the incidence of events is high they can differ quite markedly, and it is unclear which is the better parameter . We explore these issues in a cluster randomised trial, the Paramedic Practitioner Older People’s Support Trial3 . Key words: Cluster randomized trials, odds ratio, relative risk, population average models, random effects models 1 Introduction Given two groups, with probabilities of binary outcomes of π0 and π1 , the Odds Ratio (OR) of outcome in group 1 relative to group 0 is related to Relative Risk (RR) by: . If there are covariates in the model , one has to estimate π0 at some covariate combination. One can see from this relationship that if the relative risk is small, or the probability of outcome π0 is small then the odds ratio and the relative risk will be close. One can also show, using the delta method, that approximately (1). ________________________________________________________________________________ Michael J Campbell, Medical Statistics Group, ScHARR, Regent Court, 30 Regent St, Sheffield S1 4DA [email protected] 2 Note that this result is derived for non-clustered data. -

Journal of Clinical Trails Market Analysis Advances in Clinical Research and Clinical Trials 2020
Journal of Clinical Trails Market Analysis Advances in Clinical Research and Clinical Trials 2020 Raymond Chong The research report on Global Clinical Trials Market 2019 Other prominent players in the value chain include Mednet keenly analyzes significant features of the industry. The Solutions, Arisglobal, eClinForce Inc., DZS Software Solutions, DSG, analysis servers market size, latest trends, drivers, threats, Inc., Guger Technologies Inc., ICON, Plc., ChemWare Inc., and opportunities, as well as key market segments. It is based on iWeb Technologies Limited. past data and present market needs. Also, involve distinct business approaches accepted by the decision makers. Those intensify growth and make a remarkable stand in the industry. The Clinical Trials market will grow with a significant CAGR between 2019 to 2028. The report segregates the complete market on the basis of key players, geographical areas, and segments. Increasing demand for new medical equipments and medicines among end users, coupled with growing investment for research and development activities for development of effective medicines are major factors driving growth of the global clinical trials market. In addition, increasing number of individuals suffering from chronic diseases as well as changing The clinical trials market is projected to arrive at USD 1.76 conditions and nature of certain types of chronic diseases is billion by 2025, from USD 1.04 billion in 2017 growing at a another factor anticipated to support growth of the global CAGR of 6.7 percent from 2018 to 2025. An upcoming clinical trials market to significant extent. market report contains data for historic year 2016, and the base year of calculation is 2017. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Delineating Virulence of Vibrio Campbellii
www.nature.com/scientificreports OPEN Delineating virulence of Vibrio campbellii: a predominant luminescent bacterial pathogen in Indian shrimp hatcheries Sujeet Kumar1*, Chandra Bhushan Kumar1,2, Vidya Rajendran1, Nishawlini Abishaw1, P. S. Shyne Anand1, S. Kannapan1, Viswas K. Nagaleekar3, K. K. Vijayan1 & S. V. Alavandi1 Luminescent vibriosis is a major bacterial disease in shrimp hatcheries and causes up to 100% mortality in larval stages of penaeid shrimps. We investigated the virulence factors and genetic identity of 29 luminescent Vibrio isolates from Indian shrimp hatcheries and farms, which were earlier presumed as Vibrio harveyi. Haemolysin gene-based species-specifc multiplex PCR and phylogenetic analysis of rpoD and toxR identifed all the isolates as V. campbellii. The gene-specifc PCR revealed the presence of virulence markers involved in quorum sensing (luxM, luxS, cqsA), motility (faA, lafA), toxin (hly, chiA, serine protease, metalloprotease), and virulence regulators (toxR, luxR) in all the isolates. The deduced amino acid sequence analysis of virulence regulator ToxR suggested four variants, namely A123Q150 (AQ; 18.9%), P123Q150 (PQ; 54.1%), A123P150 (AP; 21.6%), and P123P150 (PP; 5.4% isolates) based on amino acid at 123rd (proline or alanine) and 150th (glutamine or proline) positions. A signifcantly higher level of the quorum-sensing signal, autoinducer-2 (AI-2, p = 2.2e−12), and signifcantly reduced protease activity (p = 1.6e−07) were recorded in AP variant, whereas an inverse trend was noticed in the Q150 variants AQ and PQ. The pathogenicity study in Penaeus (Litopenaeus) vannamei juveniles revealed that all the isolates of AQ were highly pathogenic with Cox proportional hazard ratio 15.1 to 32.4 compared to P150 variants; PP (5.4 to 6.3) or AP (7.3 to 14). -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available.