Study Design Considerations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -
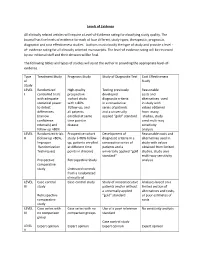
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

Meta-Analysis of Proportions
NCSS Statistical Software NCSS.com Chapter 456 Meta-Analysis of Proportions Introduction This module performs a meta-analysis of a set of two-group, binary-event studies. These studies have a treatment group (arm) and a control group. The results of each study may be summarized as counts in a 2-by-2 table. The program provides a complete set of numeric reports and plots to allow the investigation and presentation of the studies. The plots include the forest plot, radial plot, and L’Abbe plot. Both fixed-, and random-, effects models are available for analysis. Meta-Analysis refers to methods for the systematic review of a set of individual studies with the aim to combine their results. Meta-analysis has become popular for a number of reasons: 1. The adoption of evidence-based medicine which requires that all reliable information is considered. 2. The desire to avoid narrative reviews which are often misleading. 3. The desire to interpret the large number of studies that may have been conducted about a specific treatment. 4. The desire to increase the statistical power of the results be combining many small-size studies. The goals of meta-analysis may be summarized as follows. A meta-analysis seeks to systematically review all pertinent evidence, provide quantitative summaries, integrate results across studies, and provide an overall interpretation of these studies. We have found many books and articles on meta-analysis. In this chapter, we briefly summarize the information in Sutton et al (2000) and Thompson (1998). Refer to those sources for more details about how to conduct a meta- analysis. -

Contingency Tables Are Eaten by Large Birds of Prey
Case Study Case Study Example 9.3 beginning on page 213 of the text describes an experiment in which fish are placed in a large tank for a period of time and some Contingency Tables are eaten by large birds of prey. The fish are categorized by their level of parasitic infection, either uninfected, lightly infected, or highly infected. It is to the parasites' advantage to be in a fish that is eaten, as this provides Bret Hanlon and Bret Larget an opportunity to infect the bird in the parasites' next stage of life. The observed proportions of fish eaten are quite different among the categories. Department of Statistics University of Wisconsin|Madison Uninfected Lightly Infected Highly Infected Total October 4{6, 2011 Eaten 1 10 37 48 Not eaten 49 35 9 93 Total 50 45 46 141 The proportions of eaten fish are, respectively, 1=50 = 0:02, 10=45 = 0:222, and 37=46 = 0:804. Contingency Tables 1 / 56 Contingency Tables Case Study Infected Fish and Predation 2 / 56 Stacked Bar Graph Graphing Tabled Counts Eaten Not eaten 50 40 A stacked bar graph shows: I the sample sizes in each sample; and I the number of observations of each type within each sample. 30 This plot makes it easy to compare sample sizes among samples and 20 counts within samples, but the comparison of estimates of conditional Frequency probabilities among samples is less clear. 10 0 Uninfected Lightly Infected Highly Infected Contingency Tables Case Study Graphics 3 / 56 Contingency Tables Case Study Graphics 4 / 56 Mosaic Plot Mosaic Plot Eaten Not eaten 1.0 0.8 A mosaic plot replaces absolute frequencies (counts) with relative frequencies within each sample. -

A Case-Crossover Study of Sleep and Childhood Injury
A Case-Crossover Study of Sleep and Childhood Injury Francesca Valent, MD*; Silvio Brusaferro, MD*; and Fabio Barbone, MD, DrPH*‡ ABSTRACT. Objective. To evaluate the association n Italy (ϳ56 million inhabitants; 9 million chil- between sleep and wakefulness duration and childhood dren Ͻ15 years of age), ϳ500 children Ͻ15 years unintentional injury. of age are killed each year in unintentional inju- Study Design. Case-crossover study. I 1 ries. Homicides and suicides are much less frequent, Methods. Two hundred ninety-two injured children accounting for Ͻ10% of all fatal injuries among chil- who presented at the Children’s Emergency Center of dren.1 In Northern Italy, in 1995, injury mortality Udine, Italy, or their parents were interviewed after a rates (International Classification of Diseases, Ninth Re- structured questionnaire. Information was collected con- vision codes from 800 to 999) were 10 deaths/100 000 cerning sociodemographic variables, participant’s habits, and injury characteristics, including a brief description inhabitants under 1 year of age, 5/100 000 from 1 to of the accident dynamics. Sleep or wakefulness status of 4 years of age, and 6/100 000 from 5 to 14 years of the child was assessed retrospectively for each of the 48 age. Among infants, injury represented only 2% of hours before injury. For each child, we compared the 24 overall mortality, whereas in the 2 older age groups hours immediately before the injury (hours 1–24; case injuries accounted for 18% and 33% of all deaths, period) with hours 25 to 48 (control period). Nonparamet- respectively.2 Mortality, however, is just the tip of a ric tests were conducted to compare the difference of pyramid, where for each injury resulting in a death sleep duration between case and control periods. -

TUTORIAL in BIOSTATISTICS: the Self-Controlled Case Series Method
STATISTICS IN MEDICINE Statist. Med. 2005; 0:1–31 Prepared using simauth.cls [Version: 2002/09/18 v1.11] TUTORIAL IN BIOSTATISTICS: The self-controlled case series method Heather J. Whitaker1, C. Paddy Farrington1, Bart Spiessens2 and Patrick Musonda1 1 Department of Statistics, The Open University, Milton Keynes, MK7 6AA, UK. 2 GlaxoSmithKline Biologicals, Rue de l’Institut 89, B-1330 Rixensart, Belgium. SUMMARY The self-controlled case series method was developed to investigate associations between acute outcomes and transient exposures, using only data on cases, that is, on individuals who have experienced the outcome of interest. Inference is within individuals, and hence fixed covariates effects are implicitly controlled for within a proportional incidence framework. We describe the origins, assumptions, limitations, and uses of the method. The rationale for the model and the derivation of the likelihood are explained in detail using a worked example on vaccine safety. Code for fitting the model in the statistical package STATA is described. Two further vaccine safety data sets are used to illustrate a range of modelling issues and extensions of the basic model. Some brief pointers on the design of case series studies are provided. The data sets, STATA code, and further implementation details in SAS, GENSTAT and GLIM are available from an associated website. key words: case series; conditional likelihood; control; epidemiology; modelling; proportional incidence Copyright c 2005 John Wiley & Sons, Ltd. 1. Introduction The self-controlled case series method, or case series method for short, provides an alternative to more established cohort or case-control methods for investigating the association between a time-varying exposure and an outcome event. -

Supplementary File
Supplementary file Table S1. Characteristics of studies included in the systematic review. Authors, reference Type of article Number of patients Type of stroke Valderrama [1] case report 1 AIS Oxley [2] case series 5 AIS Morassi [3] case series 6 AIS (n = 4), HS (n = 2) Tunc [4] case series 4 AIS Avula [5] case series 4 AIS (n = 3), TIA (n = 1) Oliver [6] case report 1 AIS Beyrouti [7] case series 6 AIS Al Saiegh [8] case series 2 AIS (n = 1), SAH (n = 1) Gunasekaran [9] case report 1 AIS Goldberg [10] case report 1 AIS Viguier [11] case report 1 AIS Escalard [12] case series/ case control 10 AIS Wang [13] case series 5 AIS Fara [14] case series 3 AIS Moshayedi [15] case report 1 AIS Deliwala [16] case report 1 AIS Sharafi-Razavi [17] case report 1 HS retrospective cohort study (case Jain [18] 35 AIS (n = 26) HS (n = 9) control) retrospective cohort study (case Yaghi [19] 32 AIS control) AIS (n = 35), TIA (n = 5), HS Benussi [20] retrospective cohort study 43 (n = 3) Rudilosso [21] case report 1 AIS Lodigiani [22] retrospective cohort study 9 AIS Malentacchi [23] case report 1 AIS J. Clin. Med. 2020, 9, x; doi: FOR PEER REVIEW www.mdpi.com/journal/jcm J. Clin. Med. 2020, 9, x FOR PEER REVIEW 2 of 8 retrospective cohort study/case Scullen [24] 2 HS + AIS (n = 1), AIS (n = 1) series retrospective cohort study/ case AIS (n = 17), ST (n = 2), HS Sweid [25] 22 series (n = 3) AIS – acute ischemic stroke; HS – haemorrhagic stroke; SAH – subarachnoid haemorrhage; ST – sinus thrombosis; TIA-transient ischemic attack. -

Relative Risks, Odds Ratios and Cluster Randomised Trials . (1)
1 Relative Risks, Odds Ratios and Cluster Randomised Trials Michael J Campbell, Suzanne Mason, Jon Nicholl Abstract It is well known in cluster randomised trials with a binary outcome and a logistic link that the population average model and cluster specific model estimate different population parameters for the odds ratio. However, it is less well appreciated that for a log link, the population parameters are the same and a log link leads to a relative risk. This suggests that for a prospective cluster randomised trial the relative risk is easier to interpret. Commonly the odds ratio and the relative risk have similar values and are interpreted similarly. However, when the incidence of events is high they can differ quite markedly, and it is unclear which is the better parameter . We explore these issues in a cluster randomised trial, the Paramedic Practitioner Older People’s Support Trial3 . Key words: Cluster randomized trials, odds ratio, relative risk, population average models, random effects models 1 Introduction Given two groups, with probabilities of binary outcomes of π0 and π1 , the Odds Ratio (OR) of outcome in group 1 relative to group 0 is related to Relative Risk (RR) by: . If there are covariates in the model , one has to estimate π0 at some covariate combination. One can see from this relationship that if the relative risk is small, or the probability of outcome π0 is small then the odds ratio and the relative risk will be close. One can also show, using the delta method, that approximately (1). ________________________________________________________________________________ Michael J Campbell, Medical Statistics Group, ScHARR, Regent Court, 30 Regent St, Sheffield S1 4DA [email protected] 2 Note that this result is derived for non-clustered data. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Case Series: COVID-19 Infection Causing New-Onset Diabetes Mellitus?
Endocrinology & Metabolism International Journal Case Series Open Access Case series: COVID-19 infection causing new-onset diabetes mellitus? Abstract Volume 9 Issue 1 - 2021 Objectives: This paper seeks to explore the hypothesis of the potential diabetogenic effect 1 2 of SARS-COV-2 (Severe Acute respiratory syndrome coronavirus). Rujuta Katkar, Narasa Raju Madam 1Consultant Endocrinologist at Yuma Regional Medical Center, Case series presentation: We present a case series of observation among 8 patients of age USA 2 group ranging from 34 to 74 years with a BMI range of 26.61 to 53.21 Kilogram/square Hospitalist at Yuma Regional Medical Center, USA meters that developed new-onset diabetes after COVID-19 infection. Correspondence: Rujuta Katkar, MD, Consultant Severe Acute Respiratory Syndrome Coronavirus (SARS-COV-2), commonly known as Endocrinologist at Yuma Regional Medical Center, 2851 S Coronavirus or COVID-19(Coronavirus infectious disease), gains entry into the cells by Avenue B, Bldg.20, Yuma, AZ-85364, USA, Tel 2034359976, binding to the Angiotensin-converting enzyme-2(ACE-2) receptors located in essential Email metabolic tissues including the pancreas, adipose tissue, small intestine, and kidneys. Received: March 25, 2021 | Published: April 02, 2021 The evidence reviewed from the scientific literature describes how ACE 2 receptors play a role in the pathogenesis of diabetes and the plausible interaction of SARS-COV-2 with ACE 2 receptors in metabolic organs and tissues. Conclusion: The 8 patients without a past medical history of diabetes admitted with COVID-19 infection developed new-onset diabetes mellitus due to plausible interaction of SARS-COV-2 with ACE 2 receptors. -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available.