Bias in Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
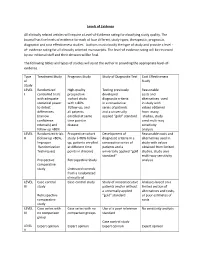
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

(HCW) Surveys in Humanitarian Contexts in Lmics
Analytics for Operations working group GUIDANCE BRIEF Guidance for Health Care Worker (HCW) Surveys in humanitarian contexts in LMICs Developed by the Analytics for Operations Working Group to support those working with communities and healthcare workers in humanitarian and emergency contexts. This document has been developed for response actors working in humanitarian contexts who seek rapid approaches to gathering evidence about the experience of healthcare workers, and the communities of which they are a part. Understanding healthcare worker experience is critical to inform and guide humanitarian programming and effective strategies to promote IPC, identify psychosocial support needs. This evidence also informs humanitarian programming that interacts with HCWs and facilities such as nutrition, health reinforcement, communication, SGBV and gender. In low- and middle-income countries (LMIC), healthcare workers (HCW) are often faced with limited resources, equipment, performance support and even formal training to provide the life-saving work expected of them. In humanitarian contexts1, where human resources are also scarce, HCWs may comprise formally trained doctors, nurses, pharmacists, dentists, allied health professionals etc. as well as community members who perform formal health worker related duties with little or no trainingi. These HCWs frequently work in contexts of multiple public health crises, including COVID-19. Their work will be affected by availability of resources (limited supplies, materials), behaviour and emotion (fear), flows of (mis)information (e.g. understanding of expected infection prevention and control (IPC) measures) or services (healthcare policies, services and use). Multiple factors can therefore impact patients, HCWs and their families, not only in terms of risk of exposure to COVID-19, but secondary health, socio-economic and psycho-social risks, as well as constraints that interrupt or hinder healthcare provision such as physical distancing practices. -

TUTORIAL in BIOSTATISTICS: the Self-Controlled Case Series Method
STATISTICS IN MEDICINE Statist. Med. 2005; 0:1–31 Prepared using simauth.cls [Version: 2002/09/18 v1.11] TUTORIAL IN BIOSTATISTICS: The self-controlled case series method Heather J. Whitaker1, C. Paddy Farrington1, Bart Spiessens2 and Patrick Musonda1 1 Department of Statistics, The Open University, Milton Keynes, MK7 6AA, UK. 2 GlaxoSmithKline Biologicals, Rue de l’Institut 89, B-1330 Rixensart, Belgium. SUMMARY The self-controlled case series method was developed to investigate associations between acute outcomes and transient exposures, using only data on cases, that is, on individuals who have experienced the outcome of interest. Inference is within individuals, and hence fixed covariates effects are implicitly controlled for within a proportional incidence framework. We describe the origins, assumptions, limitations, and uses of the method. The rationale for the model and the derivation of the likelihood are explained in detail using a worked example on vaccine safety. Code for fitting the model in the statistical package STATA is described. Two further vaccine safety data sets are used to illustrate a range of modelling issues and extensions of the basic model. Some brief pointers on the design of case series studies are provided. The data sets, STATA code, and further implementation details in SAS, GENSTAT and GLIM are available from an associated website. key words: case series; conditional likelihood; control; epidemiology; modelling; proportional incidence Copyright c 2005 John Wiley & Sons, Ltd. 1. Introduction The self-controlled case series method, or case series method for short, provides an alternative to more established cohort or case-control methods for investigating the association between a time-varying exposure and an outcome event. -

Supplementary File
Supplementary file Table S1. Characteristics of studies included in the systematic review. Authors, reference Type of article Number of patients Type of stroke Valderrama [1] case report 1 AIS Oxley [2] case series 5 AIS Morassi [3] case series 6 AIS (n = 4), HS (n = 2) Tunc [4] case series 4 AIS Avula [5] case series 4 AIS (n = 3), TIA (n = 1) Oliver [6] case report 1 AIS Beyrouti [7] case series 6 AIS Al Saiegh [8] case series 2 AIS (n = 1), SAH (n = 1) Gunasekaran [9] case report 1 AIS Goldberg [10] case report 1 AIS Viguier [11] case report 1 AIS Escalard [12] case series/ case control 10 AIS Wang [13] case series 5 AIS Fara [14] case series 3 AIS Moshayedi [15] case report 1 AIS Deliwala [16] case report 1 AIS Sharafi-Razavi [17] case report 1 HS retrospective cohort study (case Jain [18] 35 AIS (n = 26) HS (n = 9) control) retrospective cohort study (case Yaghi [19] 32 AIS control) AIS (n = 35), TIA (n = 5), HS Benussi [20] retrospective cohort study 43 (n = 3) Rudilosso [21] case report 1 AIS Lodigiani [22] retrospective cohort study 9 AIS Malentacchi [23] case report 1 AIS J. Clin. Med. 2020, 9, x; doi: FOR PEER REVIEW www.mdpi.com/journal/jcm J. Clin. Med. 2020, 9, x FOR PEER REVIEW 2 of 8 retrospective cohort study/case Scullen [24] 2 HS + AIS (n = 1), AIS (n = 1) series retrospective cohort study/ case AIS (n = 17), ST (n = 2), HS Sweid [25] 22 series (n = 3) AIS – acute ischemic stroke; HS – haemorrhagic stroke; SAH – subarachnoid haemorrhage; ST – sinus thrombosis; TIA-transient ischemic attack. -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Impact of Blinding on Estimated Treatment Effects in Randomised Clinical Trials
RESEARCH Impact of blinding on estimated treatment effects in randomised BMJ: first published as 10.1136/bmj.l6802 on 21 January 2020. Downloaded from clinical trials: meta-epidemiological study Helene Moustgaard,1-4 Gemma L Clayton,5 Hayley E Jones,5 Isabelle Boutron,6 Lars Jørgensen,4 David R T Laursen,1-4 Mette F Olsen,4 Asger Paludan-Müller,4 Philippe Ravaud,6 5,7 5,7,8 5,7 1-3 Jelena Savović, , Jonathan A C Sterne, Julian P T Higgins, Asbjørn Hróbjartsson For numbered affiliations see ABSTRACT 1 indicated exaggerated effect estimates in trials end of the article. OBJECTIVES without blinding. Correspondence to: To study the impact of blinding on estimated RESULTS H Moustgaard treatment effects, and their variation between [email protected] The study included 142 meta-analyses (1153 trials). (or @HeleneMoustgaa1 on Twitter trials; differentiating between blinding of patients, The ROR for lack of blinding of patients was 0.91 ORCID 0000-0002-7057-5251) healthcare providers, and observers; detection bias (95% credible interval 0.61 to 1.34) in 18 meta- Additional material is published and performance bias; and types of outcome (the analyses with patient reported outcomes, and 0.98 online only. To view please visit MetaBLIND study). the journal online. (0.69 to 1.39) in 14 meta-analyses with outcomes C ite this as: BMJ 2020;368:l6802 DESIGN reported by blinded observers. The ROR for lack of http://dx.doi.org/10.1136/bmj.l6802 Meta-epidemiological study. blinding of healthcare providers was 1.01 (0.84 to Accepted: 19 November 2019 DATA SOURCE 1.19) in 29 meta-analyses with healthcare provider Cochrane Database of Systematic Reviews (2013-14). -

Case Series: COVID-19 Infection Causing New-Onset Diabetes Mellitus?
Endocrinology & Metabolism International Journal Case Series Open Access Case series: COVID-19 infection causing new-onset diabetes mellitus? Abstract Volume 9 Issue 1 - 2021 Objectives: This paper seeks to explore the hypothesis of the potential diabetogenic effect 1 2 of SARS-COV-2 (Severe Acute respiratory syndrome coronavirus). Rujuta Katkar, Narasa Raju Madam 1Consultant Endocrinologist at Yuma Regional Medical Center, Case series presentation: We present a case series of observation among 8 patients of age USA 2 group ranging from 34 to 74 years with a BMI range of 26.61 to 53.21 Kilogram/square Hospitalist at Yuma Regional Medical Center, USA meters that developed new-onset diabetes after COVID-19 infection. Correspondence: Rujuta Katkar, MD, Consultant Severe Acute Respiratory Syndrome Coronavirus (SARS-COV-2), commonly known as Endocrinologist at Yuma Regional Medical Center, 2851 S Coronavirus or COVID-19(Coronavirus infectious disease), gains entry into the cells by Avenue B, Bldg.20, Yuma, AZ-85364, USA, Tel 2034359976, binding to the Angiotensin-converting enzyme-2(ACE-2) receptors located in essential Email metabolic tissues including the pancreas, adipose tissue, small intestine, and kidneys. Received: March 25, 2021 | Published: April 02, 2021 The evidence reviewed from the scientific literature describes how ACE 2 receptors play a role in the pathogenesis of diabetes and the plausible interaction of SARS-COV-2 with ACE 2 receptors in metabolic organs and tissues. Conclusion: The 8 patients without a past medical history of diabetes admitted with COVID-19 infection developed new-onset diabetes mellitus due to plausible interaction of SARS-COV-2 with ACE 2 receptors. -

Development of Validated Instruments
Development of Validated Instruments Michelle Tarver, MD, PhD Medical Officer Division of Ophthalmic and Ear, Nose, and Throat Devices Center for Devices and Radiological Health Food and Drug Administration No Financial Conflicts to Disclose 2 Overview • Define Patient Reported Outcomes (PROs) • Factors to Consider when Developing PROs • FDA Guidance for PROs • Use of PROs in FDA Clinical Trials 3 Patient Reported Outcomes (PROs) • Any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else • Can be measured in absolute terms (e.g., severity of a symptom) or as a change from a previous measure • In trials, measures the effect of a medical intervention on one or more concepts – Concept is the thing being measured (e.g., symptom, effects on function, severity of health condition) 4 Concepts a PRO May Capture • Symptoms • Symptom impact and functioning • Disability/handicap • Adverse events • Treatment tolerability • Treatment satisfaction • Health-related quality of life 5 Criteria to Consider in PRO Development • Appropriateness – Does the content address the relevant questions for the device? • Acceptability – Is the questionnaire acceptable to patients? • Feasibility – Is it easy to administer and process/analyze? • Interpretability – Are the scores interpretable? Abstracted from (1) Patient Reported Outcomes Measurement Group: University of Oxford (2) NIH PROMIS Instrument Development and Validation Standards 6 Criteria -

Title: a TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF
1 Title: A TRANSMISSION-VIRULENCE EVOLUTIONARY TRADE-OFF EXPLAINS 2 ATTENUATION OF HIV-1 IN UGANDA 3 Short title: EVOLUTION OF VIRULENCE IN HIV 4 François Blanquart1, Mary Kate Grabowski2, Joshua Herbeck3, Fred Nalugoda4, David 5 Serwadda4,5, Michael A. Eller6, 7, Merlin L. Robb6,7, Ronald Gray2,4, Godfrey Kigozi4, Oliver 6 Laeyendecker8, Katrina A. Lythgoe1,9, Gertrude Nakigozi4, Thomas C. Quinn8, Steven J. 7 Reynolds8, Maria J. Wawer2, Christophe Fraser1 8 1. MRC Centre for Outbreak Analysis and Modelling, Department of Infectious Disease 9 Epidemiology, School of Public Health, Imperial College London, United Kingdom 10 2. Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, 11 Baltimore, MD, USA 12 3. International Clinical Research Center, Department of Global Health, University of 13 Washington, Seattle, WA, USA 14 4. Rakai Health Sciences Program, Entebbe, Uganda 15 5. School of Public Health, Makerere University, Kampala, Uganda 16 6. U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, 17 MD, USA 18 7. Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA 19 8. Laboratory of Immunoregulation, Division of Intramural Research, National Institute of 20 Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA 21 9. Department of Zoology, University of Oxford, United Kingdom 22 23 Abstract 24 Evolutionary theory hypothesizes that intermediate virulence maximizes pathogen fitness as 25 a result of a trade-off between virulence and transmission, but empirical evidence remains scarce. 26 We bridge this gap using data from a large and long-standing HIV-1 prospective cohort, in 27 Uganda. -

Development of a Person-Centered Conceptual Model of Perceived Fatigability
Quality of Life Research (2019) 28:1337–1347 https://doi.org/10.1007/s11136-018-2093-z Development of a person-centered conceptual model of perceived fatigability Anna L. Kratz1 · Susan L. Murphy2 · Tiffany J. Braley3 · Neil Basu4 · Shubhangi Kulkarni1 · Jenna Russell1 · Noelle E. Carlozzi1 Accepted: 17 December 2018 / Published online: 2 January 2019 © Springer Nature Switzerland AG 2019 Abstract Purpose Perceived fatigability, reflective of changes in fatigue intensity in the context of activity, has emerged as a poten- tially important clinical outcome and quality of life indicator. Unfortunately, the nature of perceived fatigability is not well characterized. The aim of this study is to define the characteristics of fatigability through the development of a conceptual model informed by input from key stakeholders who experience fatigability, including the general population, individuals with multiple sclerosis (MS), and individuals with fibromyalgia (FM). Methods Thirteen focus groups were conducted with 101 participants; five groups with n = 44 individuals representing the general population, four groups with n = 26 individuals with MS, and four groups with n = 31 individuals with FM. Focus group data were qualitatively analyzed to identify major themes in the participants’ characterizations of perceived fatigability. Results Seven major themes were identified: general fatigability, physical fatigability, mental fatigability, emotional fati- gability, moderators of fatigability, proactive and reactive behaviors, and temporal aspects of fatigability. Relative to those in the general sample, FM or MS groups more often described experiencing fatigue as a result of cognitive activity, use of proactive behaviors to manage fatigability, and sensory stimulation as exacerbating fatigability. Conclusions Fatigability is the complex and dynamic process of the development of physical, mental, and/or emotional fatigue. -

Catalogue of Clinical Trials and Cohort Studies to Identify Biological
Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for recent HIV infection Final Report February 2010 This final report was prepared by Dr Joanne Micallef and Professor John Kaldor, The University of New South Wales, under subcontract with Family Health International, funded by the Bill and Melinda Gates Foundation under the grant titled “Development of Assays for Acute HIV Infection and Estimation and HIV Incidence in Population”. 1 Catalogue of clinical trials and cohort studies to identify biological specimens of relevance to the development of assays for acute and early HIV infection: Final Report Table of Contents Table of Contents .................................................................................................................................................................... 2 List of acronyms ...................................................................................................................................................................... 4 1 Introduction ..................................................................................................................................................................... 6 2 Methods ............................................................................................................................................................................ -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available.