Biostatistics and Epidemiology Step 1 Sample Questions Set 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
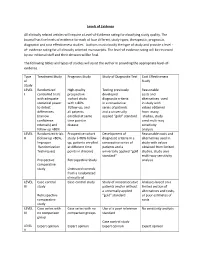
Levels of Evidence Table
Levels of Evidence All clinically related articles will require a Level-of-Evidence rating for classifying study quality. The Journal has five levels of evidence for each of four different study types; therapeutic, prognostic, diagnostic and cost effectiveness studies. Authors must classify the type of study and provide a level - of- evidence rating for all clinically oriented manuscripts. The level-of evidence rating will be reviewed by our editorial staff and their decision will be final. The following tables and types of studies will assist the author in providing the appropriate level-of- evidence. Type Treatment Study Prognosis Study Study of Diagnostic Test Cost Effectiveness of Study Study LEVEL Randomized High-quality Testing previously Reasonable I controlled trials prospective developed costs and with adequate cohort study diagnostic criteria alternatives used statistical power with > 80% in a consecutive in study with to detect follow-up, and series of patients values obtained differences all patients and a universally from many (narrow enrolled at same applied “gold” standard studies, study confidence time point in used multi-way intervals) and disease sensitivity follow up >80% analysis LEVEL Randomized trials Prospective cohort Development of Reasonable costs and II (follow up <80%, study (<80% follow- diagnostic criteria in a alternatives used in Improper up, patients enrolled consecutive series of study with values Randomization at different time patients and a obtained from limited Techniques) points in disease) universally -

The Magic of Randomization Versus the Myth of Real-World Evidence
The new england journal of medicine Sounding Board The Magic of Randomization versus the Myth of Real-World Evidence Rory Collins, F.R.S., Louise Bowman, M.D., F.R.C.P., Martin Landray, Ph.D., F.R.C.P., and Richard Peto, F.R.S. Nonrandomized observational analyses of large safety and efficacy because the potential biases electronic patient databases are being promoted with respect to both can be appreciable. For ex- as an alternative to randomized clinical trials as ample, the treatment that is being assessed may a source of “real-world evidence” about the effi- well have been provided more or less often to cacy and safety of new and existing treatments.1-3 patients who had an increased or decreased risk For drugs or procedures that are already being of various health outcomes. Indeed, that is what used widely, such observational studies may in- would be expected in medical practice, since both volve exposure of large numbers of patients. the severity of the disease being treated and the Consequently, they have the potential to detect presence of other conditions may well affect the rare adverse effects that cannot plausibly be at- choice of treatment (often in ways that cannot be tributed to bias, generally because the relative reliably quantified). Even when associations of risk is large (e.g., Reye’s syndrome associated various health outcomes with a particular treat- with the use of aspirin, or rhabdomyolysis as- ment remain statistically significant after adjust- sociated with the use of statin therapy).4 Non- ment for all the known differences between pa- randomized clinical observation may also suf- tients who received it and those who did not fice to detect large beneficial effects when good receive it, these adjusted associations may still outcomes would not otherwise be expected (e.g., reflect residual confounding because of differ- control of diabetic ketoacidosis with insulin treat- ences in factors that were assessed only incom- ment, or the rapid shrinking of tumors with pletely or not at all (and therefore could not be chemotherapy). -

(Meta-Analyses of Observational Studies in Epidemiology) Checklist
MOOSE (Meta-analyses Of Observational Studies in Epidemiology) Checklist A reporting checklist for Authors, Editors, and Reviewers of Meta-analyses of Observational Studies. You must report the page number in your manuscript where you consider each of the items listed in this checklist. If you have not included this information, either revise your manuscript accordingly before submitting or note N/A. Reporting Criteria Reported (Yes/No) Reported on Page No. Reporting of Background Problem definition Hypothesis statement Description of Study Outcome(s) Type of exposure or intervention used Type of study design used Study population Reporting of Search Strategy Qualifications of searchers (eg, librarians and investigators) Search strategy, including time period included in the synthesis and keywords Effort to include all available studies, including contact with authors Databases and registries searched Search software used, name and version, including special features used (eg, explosion) Use of hand searching (eg, reference lists of obtained articles) List of citations located and those excluded, including justification Method for addressing articles published in languages other than English Method of handling abstracts and unpublished studies Description of any contact with authors Reporting of Methods Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested Rationale for the selection and coding of data (eg, sound clinical principles or convenience) Documentation of how data were classified -

Quasi-Experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: a Systematic Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Infect Control Manuscript Author Hosp Epidemiol Manuscript Author . Author manuscript; available in PMC 2019 November 12. Published in final edited form as: Infect Control Hosp Epidemiol. 2018 February ; 39(2): 170–176. doi:10.1017/ice.2017.296. Quasi-experimental Studies in the Fields of Infection Control and Antibiotic Resistance, Ten Years Later: A Systematic Review Rotana Alsaggaf, MS, Lyndsay M. O’Hara, PhD, MPH, Kristen A. Stafford, PhD, MPH, Surbhi Leekha, MBBS, MPH, Anthony D. Harris, MD, MPH, CDC Prevention Epicenters Program Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland. Abstract OBJECTIVE.—A systematic review of quasi-experimental studies in the field of infectious diseases was published in 2005. The aim of this study was to assess improvements in the design and reporting of quasi-experiments 10 years after the initial review. We also aimed to report the statistical methods used to analyze quasi-experimental data. DESIGN.—Systematic review of articles published from January 1, 2013, to December 31, 2014, in 4 major infectious disease journals. METHODS.—Quasi-experimental studies focused on infection control and antibiotic resistance were identified and classified based on 4 criteria: (1) type of quasi-experimental design used, (2) justification of the use of the design, (3) use of correct nomenclature to describe the design, and (4) statistical methods used. RESULTS.—Of 2,600 articles, 173 (7%) featured a quasi-experimental design, compared to 73 of 2,320 articles (3%) in the previous review (P<.01). Moreover, 21 articles (12%) utilized a study design with a control group; 6 (3.5%) justified the use of a quasi-experimental design; and 68 (39%) identified their design using the correct nomenclature. -

Epidemiology and Biostatistics (EPBI) 1
Epidemiology and Biostatistics (EPBI) 1 Epidemiology and Biostatistics (EPBI) Courses EPBI 2219. Biostatistics and Public Health. 3 Credit Hours. This course is designed to provide students with a solid background in applied biostatistics in the field of public health. Specifically, the course includes an introduction to the application of biostatistics and a discussion of key statistical tests. Appropriate techniques to measure the extent of disease, the development of disease, and comparisons between groups in terms of the extent and development of disease are discussed. Techniques for summarizing data collected in samples are presented along with limited discussion of probability theory. Procedures for estimation and hypothesis testing are presented for means, for proportions, and for comparisons of means and proportions in two or more groups. Multivariable statistical methods are introduced but not covered extensively in this undergraduate course. Public Health majors, minors or students studying in the Public Health concentration must complete this course with a C or better. Level Registration Restrictions: May not be enrolled in one of the following Levels: Graduate. Repeatability: This course may not be repeated for additional credits. EPBI 2301. Public Health without Borders. 3 Credit Hours. Public Health without Borders is a course that will introduce you to the world of disease detectives to solve public health challenges in glocal (i.e., global and local) communities. You will learn about conducting disease investigations to support public health actions relevant to affected populations. You will discover what it takes to become a field epidemiologist through hands-on activities focused on promoting health and preventing disease in diverse populations across the globe. -

Observational Clinical Research
E REVIEW ARTICLE Clinical Research Methodology 2: Observational Clinical Research Daniel I. Sessler, MD, and Peter B. Imrey, PhD * † Case-control and cohort studies are invaluable research tools and provide the strongest fea- sible research designs for addressing some questions. Case-control studies usually involve retrospective data collection. Cohort studies can involve retrospective, ambidirectional, or prospective data collection. Observational studies are subject to errors attributable to selec- tion bias, confounding, measurement bias, and reverse causation—in addition to errors of chance. Confounding can be statistically controlled to the extent that potential factors are known and accurately measured, but, in practice, bias and unknown confounders usually remain additional potential sources of error, often of unknown magnitude and clinical impact. Causality—the most clinically useful relation between exposure and outcome—can rarely be defnitively determined from observational studies because intentional, controlled manipu- lations of exposures are not involved. In this article, we review several types of observa- tional clinical research: case series, comparative case-control and cohort studies, and hybrid designs in which case-control analyses are performed on selected members of cohorts. We also discuss the analytic issues that arise when groups to be compared in an observational study, such as patients receiving different therapies, are not comparable in other respects. (Anesth Analg 2015;121:1043–51) bservational clinical studies are attractive because Group, and the American Society of Anesthesiologists they are relatively inexpensive and, perhaps more Anesthesia Quality Institute. importantly, can be performed quickly if the required Recent retrospective perioperative studies include data O 1,2 data are already available. -

Guidelines for Reporting Meta-Epidemiological Methodology Research
EBM Primer Evid Based Med: first published as 10.1136/ebmed-2017-110713 on 12 July 2017. Downloaded from Guidelines for reporting meta-epidemiological methodology research Mohammad Hassan Murad, Zhen Wang 10.1136/ebmed-2017-110713 Abstract The goal is generally broad but often focuses on exam- Published research should be reported to evidence users ining the impact of certain characteristics of clinical studies on the observed effect, describing the distribu- Evidence-Based Practice with clarity and transparency that facilitate optimal tion of research evidence in a specific setting, exam- Center, Mayo Clinic, Rochester, appraisal and use of evidence and allow replication Minnesota, USA by other researchers. Guidelines for such reporting ining heterogeneity and exploring its causes, identifying are available for several types of studies but not for and describing plausible biases and providing empirical meta-epidemiological methodology studies. Meta- evidence for hypothesised associations. Unlike classic Correspondence to: epidemiological studies adopt a systematic review epidemiology, the unit of analysis for meta-epidemio- Dr Mohammad Hassan Murad, or meta-analysis approach to examine the impact logical studies is a study, not a patient. The outcomes Evidence-based Practice Center, of certain characteristics of clinical studies on the of meta-epidemiological studies are usually not clinical Mayo Clinic, 200 First Street 6–8 observed effect and provide empirical evidence for outcomes. SW, Rochester, MN 55905, USA; hypothesised associations. The unit of analysis in meta- In this guide, we adapt the items used in the PRISMA murad. mohammad@ mayo. edu 9 epidemiological studies is a study, not a patient. The statement for reporting systematic reviews and outcomes of meta-epidemiological studies are usually meta-analysis to fit the setting of meta- epidemiological not clinical outcomes. -

Top Ten Cool Things About 250B Exam 1
Cohort Studies Madhukar Pai, MD, PhD McGill University Montreal 1 Cohort: origins of the word http://www.caerleon.net/ 2 Introduction • Measurement of the occurrence of events over time is a central goal of epidemiologic research • Regardless of any particular study design or hypothesis, interest is ultimately in the disease or outcome-causing properties of factors that are antecedent to the disease or outcome. • All study designs (including case control and cross- sectional studies) are played out in some populations over time (either well defined cohorts or not) – They differ in how they acknowledge time and how they sample exposed and non-exposed as these groups develop disease over time 3 All the action happens within a “sea of person-time” in which events occur 4 Morgenstern IJE 1980 Cohort studies Intuitive approach to studying disease incidence and risk factors: 1. Start with a population at risk 2. Measure exposures and covariates at baseline 3. Follow-up the cohort over time with a) Surveillance for events or b) re-examination 4. Keep track of attrition, withdrawals, drop-outs and competing risks 5. For covariates that change over time, measure them again during follow up 6. Compare event rates in people with and without exposures of interest - Incidence Density Ratio (IDR) is the most natural and appropriate measure of effect - Adjust for confounders and compute adjusted IDR - Look for effect measure modification, if appropriate 5 Cohort studies • Can be large or small • Can be long or short duration • Can be simple or elaborate • Can look at multiple exposures and multiple outcomes • Can look at changes in exposures over time • For rare outcomes need many people and/or lengthy follow- up • Are usually very expensive because of the numbers and follow-up requirements • But once a cohort is established, can sustain research productivity for a long, long time! 6 Cohort: keeping track of people 7 Szklo & Nieto. -

Simple Linear Regression: Straight Line Regression Between an Outcome Variable (Y ) and a Single Explanatory Or Predictor Variable (X)
1 Introduction to Regression \Regression" is a generic term for statistical methods that attempt to fit a model to data, in order to quantify the relationship between the dependent (outcome) variable and the predictor (independent) variable(s). Assuming it fits the data reasonable well, the estimated model may then be used either to merely describe the relationship between the two groups of variables (explanatory), or to predict new values (prediction). There are many types of regression models, here are a few most common to epidemiology: Simple Linear Regression: Straight line regression between an outcome variable (Y ) and a single explanatory or predictor variable (X). E(Y ) = α + β × X Multiple Linear Regression: Same as Simple Linear Regression, but now with possibly multiple explanatory or predictor variables. E(Y ) = α + β1 × X1 + β2 × X2 + β3 × X3 + ::: A special case is polynomial regression. 2 3 E(Y ) = α + β1 × X + β2 × X + β3 × X + ::: Generalized Linear Model: Same as Multiple Linear Regression, but with a possibly transformed Y variable. This introduces considerable flexibil- ity, as non-linear and non-normal situations can be easily handled. G(E(Y )) = α + β1 × X1 + β2 × X2 + β3 × X3 + ::: In general, the transformation function G(Y ) can take any form, but a few forms are especially common: • Taking G(Y ) = logit(Y ) describes a logistic regression model: E(Y ) log( ) = α + β × X + β × X + β × X + ::: 1 − E(Y ) 1 1 2 2 3 3 2 • Taking G(Y ) = log(Y ) is also very common, leading to Poisson regression for count data, and other so called \log-linear" models. -

Epidemiologic Study Designs
Epidemiologic Study Designs Jacky M Jennings, PhD, MPH Associate Professor Associate Director, General Pediatrics and Adolescent Medicine Director, Center for Child & Community Health Research (CCHR) Departments of Pediatrics & Epidemiology Johns Hopkins University Learning Objectives • Identify basic epidemiologic study designs and their frequent sequence of study • Recognize the basic components • Understand the advantages and disadvantages • Appropriately select a study design Research Question & Hypotheses Analytic Study Plan Design Basic Study Designs and their Hierarchy Clinical Observation Hypothesis Descriptive Study Case-Control Study Cohort Study Randomized Controlled Trial Systematic Review Causality Adapted from Gordis, 1996 MMWR Study Design in Epidemiology • Depends on: – The research question and hypotheses – Resources and time available for the study – Type of outcome of interest – Type of exposure of interest – Ethics Study Design in Epidemiology • Includes: – The research question and hypotheses – Measures and data quality – Time – Study population • Inclusion/exclusion criteria • Internal/external validity Epidemiologic Study Designs • Descriptive studies – Seeks to measure the frequency of disease and/or collect descriptive data on risk factors • Analytic studies – Tests a causal hypothesis about the etiology of disease • Experimental studies – Compares, for example, treatments EXPOSURE Cross-sectional OUTCOME EXPOSURE OUTCOME Case-Control EXPOSURE OUTCOME Cohort TIME Cross-sectional studies • Measure existing disease and current exposure levels at one point in time • Sample without knowledge of exposure or disease • Ex. Prevalence studies Cross-sectional studies • Advantages – Often early study design in a line of investigation – Good for hypothesis generation – Relatively easy, quick and inexpensive…depends on question – Examine multiple exposures or outcomes – Estimate prevalence of disease and exposures Cross-sectional studies • Disadvantages – Cannot infer causality – Prevalent vs. -

Overview of Epidemiological Study Designs
University of Massachusetts Medical School eScholarship@UMMS PEER Liberia Project UMass Medical School Collaborations in Liberia 2018-11 Overview of Epidemiological Study Designs Richard Ssekitoleko Yale University Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/liberia_peer Part of the Clinical Epidemiology Commons, Epidemiology Commons, Family Medicine Commons, Infectious Disease Commons, Medical Education Commons, and the Quantitative, Qualitative, Comparative, and Historical Methodologies Commons Repository Citation Ssekitoleko R. (2018). Overview of Epidemiological Study Designs. PEER Liberia Project. https://doi.org/ 10.13028/fp3z-mv12. Retrieved from https://escholarship.umassmed.edu/liberia_peer/5 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in PEER Liberia Project by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Overview of Epidemiological study Designs Richard Ssekitoleko Department of Global Health Yale University 1.1 Objectives • Understand the different epidemiological study types • Get to know what is involved in each type of study • Understand the strengths and Limitations of each study type Key terms • Population • Consists of all elements and is the group from which a sample is drawn • A sample is a subset of a population • Parameters • Summary data from a population • Statistics • Summary data from a sample • Validity • Extent to which a conclusion or statistic is well-founded and likely corresponds accurately to the parameter. Hierarchy of Evidence Study type Observational Interventional Descriptive Experiment Ecological Randomized Controlled Trial Cross-sectional Case-control Cohort Overview of Epidemiologic Study Designs Validity *anecdotes Cost Understanding What Physicians Mean: In my experience … once. -

A Systematic Review of Quasi-Experimental Study Designs in the Fields of Infection Control and Antibiotic Resistance
INVITED ARTICLE ANTIMICROBIAL RESISTANCE George M. Eliopoulos, Section Editor A Systematic Review of Quasi-Experimental Study Designs in the Fields of Infection Control and Antibiotic Resistance Anthony D. Harris,1,2 Ebbing Lautenbach,3,4 and Eli Perencevich1,2 1 2 Division of Health Care Outcomes Research, Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine, and Veterans Downloaded from https://academic.oup.com/cid/article/41/1/77/325424 by guest on 24 September 2021 Affairs Maryland Health Care System, Baltimore, Maryland; and 3Division of Infectious Diseases, Department of Medicine, and 4Department of Biostatistics and Epidemiology, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania We performed a systematic review of articles published during a 2-year period in 4 journals in the field of infectious diseases to determine the extent to which the quasi-experimental study design is used to evaluate infection control and antibiotic resistance. We evaluated studies on the basis of the following criteria: type of quasi-experimental study design used, justification of the use of the design, use of correct nomenclature to describe the design, and recognition of potential limitations of the design. A total of 73 articles featured a quasi-experimental study design. Twelve (16%) were associated with a quasi-exper- imental design involving a control group. Three (4%) provided justification for the use of the quasi-experimental study design. Sixteen (22%) used correct nomenclature to describe the study. Seventeen (23%) mentioned at least 1 of the potential limitations of the use of a quasi-experimental study design.