Biological Oceanography, Biogeochemical Cycles, and Pelagic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

The Effects of Detritus Input on Soil Organic Matter Content and Carbon Dioxide Emission in a Central European Deciduous Forest
Acta Silv. Lign. Hung., Vol. 7 (2011) 87–96 The Effects of Detritus Input on Soil Organic Matter Content and Carbon Dioxide Emission in a Central European Deciduous Forest a* b c István FEKETE – Zsolt KOTROCZÓ – Csaba VARGA – b b Zsuzsa VERES – János Attila TÓTH a Institute of Environmental Science, College of Nyíregyháza, Nyíregyháza, Hungary b Department of Ecology, University of Debrecen, Debrecen, Hungary c Department of Land and Environmental Management, College of Nyíregyháza, Nyíregyháza, Hungary Abstract – A major objective of our research was to survey soil biological activity and organic matter content reduction in a Central European oak forest during treatments of various detritus inputs within the Síkf őkút DIRT ( Detritus Input and Removal Treatments ) Project. Beside the control, three detritus removal and two detritus duplication treatments were applied. Our examinations have proven that soil organic matter content declined relatively fast in detritus removal treatments. The reduction was especially remarkable in root detritus removal treatments, where – due to the lack of transpiration – soils were moister during the whole year than in the other treatments. The higher moisture content, despite of the reduction of detritus input, produced an intense soil respiration. This can be explained by the fact that decomposing organisms have increased the use of soil organic matter. Detritus input reduction had a significantly greater effect on soil respiration and organic matter content than detritus input duplication of the same extent. The latter did not cause any significant change compared to the control. litter manipulation / soil respiration / DIRT Project / humus content / climate change Kivonat – Az avarinput hatása a talaj szerves anyag tartalmára és szén-dioxid kibocsátására egy Közép-európai lombhullató erd őben. -

Host-Secreted Antimicrobial Peptide Enforces Symbiotic Selectivity in Medicago Truncatula
Host-secreted antimicrobial peptide enforces symbiotic selectivity in Medicago truncatula Qi Wanga, Shengming Yanga, Jinge Liua, Kata Terecskeib, Edit Ábrahámb, Anikó Gombárc, Ágota Domonkosc, Attila Szucs} b, Péter Körmöczib, Ting Wangb, Lili Fodorc, Linyong Maod,e, Zhangjun Feid,e, Éva Kondorosib,1, Péter Kalóc, Attila Keresztb, and Hongyan Zhua,1 aDepartment of Plant and Soil Sciences, University of Kentucky, Lexington, KY 40546; bInstitute of Biochemistry, Biological Research Center, Szeged 6726, Hungary; cNational Agricultural Research and Innovation Centre, Agricultural Biotechnology Institute, Gödöllo} 2100, Hungary; dBoyce Thompson Institute for Plant Research, Cornell University, Ithaca, NY 14853; and eU.S. Department of Agriculture–Agricultural Research Service Robert W. Holley Center for Agriculture and Health, Cornell University, Ithaca, NY 14853 Contributed by Éva Kondorosi, February 14, 2017 (sent for review January 17, 2017; reviewed by Rebecca Dickstein and Julia Frugoli) Legumes engage in root nodule symbioses with nitrogen-fixing effectors or microbe-associated molecular patterns (MAMPs) soil bacteria known as rhizobia. In nodule cells, bacteria are enclosed such as surface polysaccharides to facilitate their invasion of the in membrane-bound vesicles called symbiosomes and differentiate host (7, 8). Therefore, effector- or MAMP-triggered plant im- into bacteroids that are capable of converting atmospheric nitrogen munity mediated by intracellular nucleotide binding/leucine-rich into ammonia. Bacteroid differentiation -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
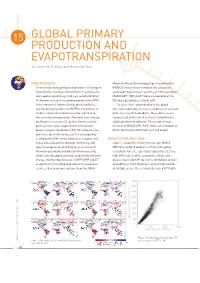
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Fractionation and Characterization of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis
Fractionation and Characterization of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1817-E Fractionation and Characterzation of Natural Organic Matter from Certain Rivers and Soils by Free-Flow Electrophoresis By J. A. LEENHEER and R. L. MALCOLM ORGANIC SUBSTANCES IN WATER GEOLOGICAL SURVEY WATER-SUPPLY PAPER 1817-E UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1973 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress catalog-card No. 73-600209 For sale by the Superintendent of Documents, U. S. Government Printing Office Washington, D. C. 20402 - Price 35 cents (paper cov«?r) Stock No. 2401-02400 CONTENTS Page Abstract ___________________________________________ El Introduction __________________________________ __ 1 Methods and materials __________________________________ 3 Sample preparation ______ _______ _ __ 3 Sample description _________________________________ 3 Separation procedure _______ ___________ __ 4 Assay of fractions ___________________________________ 4 Results and discussion _ _______________ 5 Conclusions ________________________________________ 12 References _____________________________________ __ 14 ILLUSTRATIONS Page FIGURES 1-3. Graphs showing: 1. Organic-carbon and optical-density electrophoretic fraction- ation curves for organic materials from soil and from river water ___________________________ E6 2. Organic-carbon and polysaccharide electrophoretic -

Determining the Fraction of Organic Carbon RISC Nondefault Option
Indiana Department of Environmental Management Office of Land Quality 100 N. Senate Indianapolis, IN 46204-2251 GUIDANCE OLQ PH: (317) 232-8941 Indiana Department of Environmental Management Office of Land Quality Determining the Fraction of Organic Carbon RISC Nondefault Option Background Soil can be a complex mixture of mineral-derived compounds and organic matter. The ratios of each component can vary widely depending on the type of soil being investigated. Soil organic matter is a term used by agronomists for the total organic portion of the soil and is derived from decomposed plant matter, microorganisms, and animal residues. The decomposition process can create complex high molecular weight biopolymers (e.g., humic acid) as well as simpler organic compounds (decomposed lignin or cellulose). Only the simpler organic compounds contribute to the fraction of organic carbon. There is not a rigorous definition of the fraction of organic carbon (Foc). However, it can be thought of as the portion of the organic matter that is available to adsorb the organic contaminants of concern. The higher the organic carbon content, the more organic chemicals may be adsorbed to the soil and the less of those chemicals will be available to leach to the ground water. A nondefault option in the Risk Integrated System of Closure (RISC) is to use Foc in the Soil to Ground Water Partitioning Model to calculate a site specific migration to ground water closure level. In the Soil to Ground Water Partition Model, the coefficient, Kd, for organic compounds is the Foc multiplied by the chemical-specific soil organic carbon water partition coefficient, Koc. -

Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas
microorganisms Article Characteristic Microbiomes Correlate with Polyphosphate Accumulation of Marine Sponges in South China Sea Areas 1 1, 1 1 2, 1,3, Huilong Ou , Mingyu Li y, Shufei Wu , Linli Jia , Russell T. Hill * and Jing Zhao * 1 College of Ocean and Earth Science of Xiamen University, Xiamen 361005, China; [email protected] (H.O.); [email protected] (M.L.); [email protected] (S.W.); [email protected] (L.J.) 2 Institute of Marine and Environmental Technology, University of Maryland Center for Environmental Science, Baltimore, MD 21202, USA 3 Xiamen City Key Laboratory of Urban Sea Ecological Conservation and Restoration (USER), Xiamen University, Xiamen 361005, China * Correspondence: [email protected] (J.Z.); [email protected] (R.T.H.); Tel.: +86-592-288-0811 (J.Z.); Tel.: +(410)-234-8802 (R.T.H.) The author contributed equally to the work as co-first author. y Received: 24 September 2019; Accepted: 25 December 2019; Published: 30 December 2019 Abstract: Some sponges have been shown to accumulate abundant phosphorus in the form of polyphosphate (polyP) granules even in waters where phosphorus is present at low concentrations. But the polyP accumulation occurring in sponges and their symbiotic bacteria have been little studied. The amounts of polyP exhibited significant differences in twelve sponges from marine environments with high or low dissolved inorganic phosphorus (DIP) concentrations which were quantified by spectral analysis, even though in the same sponge genus, e.g., Mycale sp. or Callyspongia sp. PolyP enrichment rates of sponges in oligotrophic environments were far higher than those in eutrophic environments. -

Human Alteration of the Global Nitrogen Cycle
What is Nitrogen? Human Alteration of the Nitrogen is the most abundant element in Global Nitrogen Cycle the Earth’s atmosphere. Nitrogen makes up 78% of the troposphere. Nitrogen cannot be absorbed directly by the plants and animals until it is converted into compounds they can use. This process is called the Nitrogen Cycle. Heather McGraw, Mandy Williams, Suzanne Heinzel, and Cristen Whorl, Give SIUE Permission to Put Our Presentation on E-reserve at Lovejoy Library. The Nitrogen Cycle How does the nitrogen cycle work? Step 1- Nitrogen Fixation- Special bacteria convert the nitrogen gas (N2 ) to ammonia (NH3) which the plants can use. Step 2- Nitrification- Nitrification is the process which converts the ammonia into nitrite ions which the plants can take in as nutrients. Step 3- Ammonification- After all of the living organisms have used the nitrogen, decomposer bacteria convert the nitrogen-rich waste compounds into simpler ones. Step 4- Denitrification- Denitrification is the final step in which other bacteria convert the simple nitrogen compounds back into nitrogen gas (N2 ), which is then released back into the atmosphere to begin the cycle again. How does human intervention affect the nitrogen cycle? Nitric Oxide (NO) is released into the atmosphere when any type of fuel is burned. This includes byproducts of internal combustion engines. Production and Use of Nitrous Oxide (N2O) is released into the atmosphere through Nitrogen Fertilizers bacteria in livestock waste and commercial fertilizers applied to the soil. Removing nitrogen from the Earth’s crust and soil when we mine nitrogen-rich mineral deposits. Discharge of municipal sewage adds nitrogen compounds to aquatic ecosystems which disrupts the ecosystem and kills fish. -

Biological Oceanography - Legendre, Louis and Rassoulzadegan, Fereidoun
OCEANOGRAPHY – Vol.II - Biological Oceanography - Legendre, Louis and Rassoulzadegan, Fereidoun BIOLOGICAL OCEANOGRAPHY Legendre, Louis and Rassoulzadegan, Fereidoun Laboratoire d'Océanographie de Villefranche, France. Keywords: Algae, allochthonous nutrient, aphotic zone, autochthonous nutrient, Auxotrophs, bacteria, bacterioplankton, benthos, carbon dioxide, carnivory, chelator, chemoautotrophs, ciliates, coastal eutrophication, coccolithophores, convection, crustaceans, cyanobacteria, detritus, diatoms, dinoflagellates, disphotic zone, dissolved organic carbon (DOC), dissolved organic matter (DOM), ecosystem, eukaryotes, euphotic zone, eutrophic, excretion, exoenzymes, exudation, fecal pellet, femtoplankton, fish, fish lavae, flagellates, food web, foraminifers, fungi, harmful algal blooms (HABs), herbivorous food web, herbivory, heterotrophs, holoplankton, ichthyoplankton, irradiance, labile, large planktonic microphages, lysis, macroplankton, marine snow, megaplankton, meroplankton, mesoplankton, metazoan, metazooplankton, microbial food web, microbial loop, microheterotrophs, microplankton, mixotrophs, mollusks, multivorous food web, mutualism, mycoplankton, nanoplankton, nekton, net community production (NCP), neuston, new production, nutrient limitation, nutrient (macro-, micro-, inorganic, organic), oligotrophic, omnivory, osmotrophs, particulate organic carbon (POC), particulate organic matter (POM), pelagic, phagocytosis, phagotrophs, photoautotorphs, photosynthesis, phytoplankton, phytoplankton bloom, picoplankton, plankton, -

Productivity and Sustainable Management of the Humboldt Current Large Marine Ecosystem Under Climate Change
Environmental Development 17 (2016) 126–144 Contents lists available at ScienceDirect Environmental Development journal homepage: www.elsevier.com/locate/envdev Productivity and Sustainable Management of the Humboldt Current Large Marine Ecosystem under climate change Dimitri Gutiérrez a,c,n, Michael Akester b,n, Laura Naranjo b a Dirección General de Investigaciones en Oceanografía y Cambio Climático, Instituto del Mar del Perú, IMARPE, Peru b Global Environment Facility (GEF)-UNDP Humboldt Current LME Project, Chile-Peru c Universidad Peruana Cayetano Heredia, Programa de Maestría en Ciencias del Mar, Lima, Peru article info abstract Article history: The Humboldt Current Large Marine Ecosystem (HCLME) covers 95% of the southeast Received 14 September 2015 Pacific seaboard of which the area of influence from the Humboldt Current and associated Received in revised form upwelling areas in the Humboldt Current System (HCS) stretches from around 4° to 40° 4 November 2015 south. Global warming will likely affect marine circulation and land-atmosphere-ocean Accepted 5 November 2015 exchanges at the regional level, affecting the productivity and biodiversity patterns along the HCLME. The expected decrease of upwelling productivity in the HCS could be am- Keywords: plified by worldwide trends of oxygen depletion and lower pH. In addition, higher fre- Humboldt-current quency of extreme climatic events, such as El Niño in a warmer ocean, might augment the Coastal-upwelling risks for the recruitment success of anchovy and other short-lived fish resources, espe- Productivity cially in the Northern HCLME. A range of non-climatic anthropogenic stressors also Fisheries Climate-change combines to reduce productivity and biomass yields.