Calcium Carbonate Production Response to Future Ocean Warming and Acidification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Coccolith Dissolution Within Copepod Guts Affects Fecal Pellet Density And
www.nature.com/scientificreports OPEN Coccolith dissolution within copepod guts afects fecal pellet density and sinking rate Received: 17 January 2018 Meredith M. White1,2, Jesica D. Waller1, Laura C. Lubelczyk1, David T. Drapeau1, Accepted: 13 June 2018 Bruce C. Bowler1, William M. Balch1 & David M. Fields1 Published: xx xx xxxx The most common biomineral produced in the contemporary ocean is calcium carbonate, including the polymorph calcite produced by coccolithophores. The surface waters of the ocean are supersaturated with respect to calcium carbonate. As a result, particulate inorganic carbon (PIC), such as calcite coccoliths, is not expected thermodynamically to dissolve in waters above the lysocline (~4500– 6000 m). However, observations indicate that up to 60–80% of calcium carbonate is lost in the upper 500–1000 m of the ocean. This is hypothesized to occur in microenvironments with reduced saturation states, such as zooplankton guts. Using a new application of the highly precise 14C microdifusion technique, we show that following a period of starvation, up to 38% of ingested calcite dissolves in copepod guts. After continued feeding, our data show the gut becomes increasingly bufered, which limits further dissolution; this has been termed the Tums hypothesis (after the drugstore remedy for stomach acid). As less calcite dissolves in the gut and is instead egested in fecal pellets, the fecal pellet sinking rates double, with corresponding increases in pellet density. Our results empirically demonstrate that zooplankton guts can facilitate calcite dissolution above the chemical lysocline, and that carbon export through fecal pellet production is variable, based on the feeding history of the copepod. -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
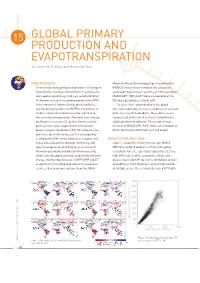
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Oregon Department of Human Services HEALTH EFFECTS INFORMATION
Oregon Department of Human Services Office of Environmental Public Health (503) 731-4030 Emergency 800 NE Oregon Street #604 (971) 673-0405 Portland, OR 97232-2162 (971) 673-0457 FAX (971) 673-0372 TTY-Nonvoice TECHNICAL BULLETIN HEALTH EFFECTS INFORMATION Prepared by: Department of Human Services ENVIRONMENTAL TOXICOLOGY SECTION Office of Environmental Public Health OCTOBER, 1998 CALCIUM CARBONATE "lime, limewater” For More Information Contact: Environmental Toxicology Section (971) 673-0440 Drinking Water Section (971) 673-0405 Technical Bulletin - Health Effects Information CALCIUM CARBONATE, "lime, limewater@ Page 2 SYNONYMS: Lime, ground limestone, dolomite, sugar lime, oyster shell, coral shell, marble dust, calcite, whiting, marl dust, putty dust CHEMICAL AND PHYSICAL PROPERTIES: - Molecular Formula: CaCO3 - White solid, crystals or powder, may draw moisture from the air and become damp on exposure - Odorless, chalky, flat, sweetish flavor (Do not confuse with "anhydrous lime" which is a special form of calcium hydroxide, an extremely caustic, dangerous product. Direct contact with it is immediately injurious to skin, eyes, intestinal tract and respiratory system.) WHERE DOES CALCIUM CARBONATE COME FROM? Calcium carbonate can be mined from the earth in solid form or it may be extracted from seawater or other brines by industrial processes. Natural shells, bones and chalk are composed predominantly of calcium carbonate. WHAT ARE THE PRINCIPLE USES OF CALCIUM CARBONATE? Calcium carbonate is an important ingredient of many household products. It is used as a whitening agent in paints, soaps, art products, paper, polishes, putty products and cement. It is used as a filler and whitener in many cosmetic products including mouth washes, creams, pastes, powders and lotions. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Ocean Acidification and Oceanic Carbon Cycling 13
Ocean Acidification and Oceanic Carbon Cycling 13 Dieter A. Wolf-Gladrow and Bjo¨rn Rost Contents Atmospheric CO2 ............................................................................... 104 Air-Sea CO2 Exchange and Ocean Carbonate Chemistry ..................................... 104 Rate of Surface Ocean Acidification and Regional Differences .. ........................... 104 The Physical Carbon Pump and Climate Change .............................................. 105 Impact of Ocean Acidification on Marine Organisms and Ecosystems ....................... 106 The Biological Carbon Pump and Climate Change ............................................ 107 Take-Home Message ............................................................................ 108 References ....................................................................................... 109 Abstract The concentration of atmospheric CO2 is increasing due to emissions from burning of fossil fuels and changes in land use. Part of this “anthropogenic CO2” invades the oceans causing a decrease of seawater pH; this process is called “ocean acidification.” The lowered pH, but also the concomitant changes in other properties of the carbonate system, affects marine life and the cycling of carbon in the ocean. Keywords Anthropogenic CO2 • Seawater acidity • Saturation state • Climate change • Physical carbon pump • Global warming • Biological carbon pumps • Phyto- plankton • Primary production • Calcification D.A. Wolf-Gladrow (*) • B. Rost Alfred Wegener Institute, Helmholtz Centre -

Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Silicate Weathering in Anoxic Marine Sediment As a Requirement for Authigenic Carbonate Burial T
Earth-Science Reviews 200 (2020) 102960 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Silicate weathering in anoxic marine sediment as a requirement for authigenic carbonate burial T Marta E. Torresa,*, Wei-Li Hongb, Evan A. Solomonc, Kitty Millikend, Ji-Hoon Kime, James C. Samplef, Barbara M.A. Teichertg, Klaus Wallmannh a College of Earth, Ocean and Atmospheric Science, Oregon State University, Corvallis OR 97331, USA b Geological Survey of Norway, Trondheim, Norway c School of Oceanography, University of Washington, Seattle, WA 98195, USA d Bureau of Economic Geology, University of Texas at Austin, Austin, TX 78713, USA e Petroleum and Marine Research Division, Korea Institute of Geoscience and Mineral Resources, Daejeon, South Korea f School of Earth and Sustainability, Northern Arizona University, Flagstaff, AZ 86011, USA g Geologisch-Paläontologisches Institut, Wilhelms-Universität Münster, Corrensstr. 24, 48149 Münster, Germany h IFM-GEOMAR Leibniz Institute of Marine Sciences, Wischhofstrasse 1-3, 24148 Kiel, Germany ARTICLE INFO ABSTRACT Keywords: We emphasize the importance of marine silicate weathering (MSiW) reactions in anoxic sediment as funda- Silicate weathering mental in generating alkalinity and cations needed for carbonate precipitation and preservation along con- Authigenic carbonate tinental margins. We use a model that couples thermodynamics with aqueous geochemistry to show that the CO2 Organogenic dolomite released during methanogenesis results in a drop in pH to 6.0; unless these protons are buffered by MSiW, Alkalinity carbonate minerals will dissolve. We present data from two regions: the India passive margin and the active Carbon cycling subduction zone off Japan, where ash and/or rivers supply the reactive silicate phase, as reflected in strontium isotope data. -

Interaction of Calcium Supplementation and Nonsteroidal Anti-Inflammatory Drugs and the Risk of Colorectal Adenomas
2353 Interaction of Calcium Supplementation and Nonsteroidal Anti-inflammatory Drugs and the Risk of Colorectal Adenomas Maria V. Grau,1 John A. Baron,1,2 Elizabeth L. Barry,1 Robert S. Sandler,3 Robert W. Haile,4 Jack S. Mandel,5 and Bernard F. Cole1 Departments of 1Community and Family Medicine and 2Medicine, Dartmouth Medical School, Lebanon, New Hamsphire; 3Department of Medicine, University of North Carolina, Chapel Hill, North Carolina; 4Department of Preventive Medicine, University of Southern California, Los Angeles, California; and 5Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia Abstract Background: Calcium and aspirin have both been found to be Results: In the Calcium Trial, subjects randomized to calcium chemopreventive against colorectal neoplasia. However, the who also were frequent users of NSAIDs had a reduction joint effect of the two agents has not been well investigated. of risk for advanced adenomas of 65% [adjusted risk ratio Methods: To explore the separate and joint effects of calcium (RR), 0.35; 95% confidence interval (95% CI), 0.13-0.96], and and aspirin/nonsteroidal anti-inflammatory drugs (NSAID), there was a highly significant statistical interaction between we used data from two large randomized clinical trials calcium treatment and frequent NSAID use (Pinteraction = among patients with a recent history of colorectal adenomas. 0.01). Similarly, in the Aspirin Trial, 81 mg aspirin and In the Calcium Polyp Prevention Study, 930 eligible subjects calcium supplement use together conferred a risk reduction were randomized to receive placebo or 1,200 mg of elemental of 80% for advanced adenomas (adjusted RR, 0.20; 95% CI, calcium daily for 4 years. -

Some Drugs Are Excluded from Medicare Part D, but Are Covered by Your Medicaid Benefits Under the Healthpartners® MSHO Plan (HMO)
Some drugs are excluded from Medicare Part D, but are covered by your Medicaid benefits under the HealthPartners® MSHO Plan (HMO). These drugs include some over‐the‐counter (OTC) items, vitamins, and cough and cold medicines. If covered, these drugs will have no copay and will not count toward your total drug cost. For questions, please call Member Services at 952‐967‐7029 or 1‐888‐820‐4285. TTY members should call 952‐883‐6060 or 1‐800‐443‐0156. From October 1 through February 14, we take calls from 8 a.m. to 8 p.m., seven days a week. You’ll speak with a representative. From February 15 to September 30, call us 8 a.m. to 8 p.m. Monday through Friday to speak with a representative. On Saturdays, Sundays and holidays, you can leave a message and we’ll get back to you within one business day. Drug Description Strength 3 DAY VAGINAL 4% 5‐HYDROXYTRYPTOPHAN 50 MG ABSORBASE ACETAMINOPHEN 500 MG ACETAMINOPHEN 120MG ACETAMINOPHEN 325 MG ACETAMINOPHEN 650MG ACETAMINOPHEN 80 MG ACETAMINOPHEN 650 MG ACETAMINOPHEN 160 MG/5ML ACETAMINOPHEN 500 MG/5ML ACETAMINOPHEN 160 MG/5ML ACETAMINOPHEN 500MG/15ML ACETAMINOPHEN 100 MG/ML ACETAMINOPHEN 500 MG ACETAMINOPHEN 325 MG ACETAMINOPHEN 500 MG ACETAMINOPHEN 80 MG ACETAMINOPHEN 100.00% ACETAMINOPHEN 80 MG ACETAMINOPHEN 160 MG ACETAMINOPHEN 80MG/0.8ML ACETAMINOPHEN‐BUTALBITAL 50MG‐325MG ACNE CLEANSING PADS 2% ACNE TREATMENT,EXTRA STRENGTH 10% ACT ANTI‐CAVITY MOUTH RINSE 0.05% Updated 12/01/2012 ACTICAL ACTIDOSE‐AQUA 50G/240ML ACTIDOSE‐AQUA 15G/72ML ACTIDOSE‐AQUA 25G/120ML ACTIVATED CHARCOAL 25 G ADEKS 7.5 MG