Understanding the Ocean's Biological Carbon Pump in the Past: Do We Have the Right Tools?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Total Organic Carbon (TOC) Guidance Manual
September 2002 RG-379 (Revised) Total Organic Carbon (TOC) Guidance Manual Water Supply Division printed on recycled paper TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Total Organic Carbon (TOC) Guidance Manual Prepared by Water Supply Division RG-379 (Revised) September 2002 Robert J. Huston, Chairman R. B. “Ralph” Marquez, Commissioner Kathleen Hartnett White, Commissioner Jeffrey A. Saitas, Executive Director Authorization to use or reproduce any original material contained in this publication—that is, not obtained from other sources—is freely granted. The commission would appreciate acknowledgment. Copies of this publication are available for public use through the Texas State Library, other state depository libraries, and the TCEQ Library, in compli- ance with state depository law. For more information on TCEQ publications call 512/239-0028 or visit our Web site at: www.tceq.state.tx.us/publications Published and distributed by the Texas Commission on Environmental Quality PO Box 13087 Austin TX 78711-3087 The Texas Commission on Environmental Quality was formerly called the Texas Natural Resource Conservation Commission. The TCEQ is an equal opportunity/affirmative action employer. The agency does not allow discrimination on the basis of race, color, religion, national origin, sex, disability, age, sexual orientation or veteran status. In compliance with the Americans with Disabilities Act, this document may be requested in alternate formats by contacting the TCEQ at 512/239-0028, Fax 239-4488, or 1-800-RELAY-TX (TDD), or by writing -

Estimation of Atmospheric Total Organic Carbon (TOC) – Paving The
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1055 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 12 October 2018 c Author(s) 2018. CC BY 4.0 License. Estimation of atmospheric total organic carbon (TOC) – paving the path towards carbon budget closure Mingxi Yang1*, Zoë L. Fleming2& 5 1 Plymouth Marine Laboratory, Plymouth, United Kingdom 2 National Centre for Atmospheric Science (NCAS), Department of Chemistry, University of Leicester, UK, United Kingdom * Correspondence to M. Yang ([email protected]) & Now at Center for Climate and Resilience Research (CR2), Departamento de Geofísica, Universidad de Chile, Santiago, Chile 10 Abstract. The atmosphere contains a rich variety of reactive organic compounds, including gaseous volatile organic carbon (VOCs), carbonaceous aerosols, and other organic compounds at varying volatility. Here we present measurements of atmospheric non-methane total organic carbon plus carbon monoxide (TOC+CO) during August-September 2016 from a coastal 15 city in the southwest United Kingdom. TOC+CO was substantially elevated during the day on weekdays (occasionally over 2 ppm C) as a result of local anthropogenic activity. On weekends and holidays, with a mean (standard error) of 102 (8) ppb C, TOC+CO was lower and showed much less diurnal variability. Excluding weekday daytime, TOC+CO was significantly lower when winds were coming off the Atlantic Ocean than when winds were coming off land. By subtracting the estimated CO from TOC+CO, we constrain the mean (uncertainty) TOC in marine air to be around 19 (±≥8) ppb C during this period. A proton- 20 transfer-reaction mass spectrometer (PTR-MS) was deployed at the same time, detecting a large range of organic compounds (oxygenated VOCs, biogenic VOCs, aromatics, dimethyl sulfide). -

Coccolith Dissolution Within Copepod Guts Affects Fecal Pellet Density And
www.nature.com/scientificreports OPEN Coccolith dissolution within copepod guts afects fecal pellet density and sinking rate Received: 17 January 2018 Meredith M. White1,2, Jesica D. Waller1, Laura C. Lubelczyk1, David T. Drapeau1, Accepted: 13 June 2018 Bruce C. Bowler1, William M. Balch1 & David M. Fields1 Published: xx xx xxxx The most common biomineral produced in the contemporary ocean is calcium carbonate, including the polymorph calcite produced by coccolithophores. The surface waters of the ocean are supersaturated with respect to calcium carbonate. As a result, particulate inorganic carbon (PIC), such as calcite coccoliths, is not expected thermodynamically to dissolve in waters above the lysocline (~4500– 6000 m). However, observations indicate that up to 60–80% of calcium carbonate is lost in the upper 500–1000 m of the ocean. This is hypothesized to occur in microenvironments with reduced saturation states, such as zooplankton guts. Using a new application of the highly precise 14C microdifusion technique, we show that following a period of starvation, up to 38% of ingested calcite dissolves in copepod guts. After continued feeding, our data show the gut becomes increasingly bufered, which limits further dissolution; this has been termed the Tums hypothesis (after the drugstore remedy for stomach acid). As less calcite dissolves in the gut and is instead egested in fecal pellets, the fecal pellet sinking rates double, with corresponding increases in pellet density. Our results empirically demonstrate that zooplankton guts can facilitate calcite dissolution above the chemical lysocline, and that carbon export through fecal pellet production is variable, based on the feeding history of the copepod. -

E.’S Responses ~(Continued)
: I’able K-3. ,Scnping stat~nts and EIS sectimns or .~E.’s responses ~(continued) COnunent scoping nuinber :Stateilmnt topic EIS section or DOE comnt h at this tti want to reiter&e that the Enviro~ntal l~act Statement should not represent. a legalistic charade ..but a sin. cere commitment to seek and evaluate pertinent in for~t ion. Obviously, any Envirocnnental. Assemnt- which led to the Find- ing of No Significant .l~act needs to be reviewed, evaluated and expanded upon, with full regard to the in~t of a broad range of interests, incl”di”g state agenciea,. the: academic comunit y, public interest groups, and private c iti-zens. We muld like to offer some co-”ts: on the in format io” euppl=ied by 00E relative to the probable contents. of the EIS. In the category of production alternatives: : It wuld seem L Need .Section 1.1 ., important tO. re-evaluate the ,need for i~reased protict ion,~nd %e Commnt D1 make every attempt to ::scale dom those need8. It is ine8ca~ able that the quest ion. af the.,need .to prodca plutoniun ia part of the greater ongoing natic,”al.?security debate. If it is indeed. essential that plutoniun: probct ion be stepped up, the Alternatives Saction 2.1 viable? alternatives should be thoroughly explored in the EIS. In the category of socioeconamics: A broad consideration of %cioeconomic .%ction 5.2.1 the state needs to be incorporated, beyond the immdiate jobs effects :-at SiU during construction and as .an ongoing operation. South Carolina has trenmndous potential for no-nuclear economic. -

Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Require
Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jorge Andres Villa Betancur Graduate Program in Environmental Science The Ohio State University 2014 Dissertation Committee: William J. Mitsch, Co-advisor Gil Bohrer, Co-advisor James Bauer Jay Martin Copyrighted by Jorge Andres Villa Betancur 2014 Abstract Emission and uptake of greenhouse gases and the production and transport of dissolved organic matter in different wetland plant communities are key wetland functions determining two important ecosystem services, climate regulation and nutrient cycling. The objective of this dissertation was to study the variation of methane emissions, carbon sequestration and exports of dissolved organic carbon in wetland plant communities of a subtropical climate in south Florida. The plant communities selected for the study of methane emissions and carbon sequestration were located in a natural wetland landscape and corresponded to a gradient of inundation duration. Going from the wettest to the driest conditions, the communities were designated as: deep slough, bald cypress, wet prairie, pond cypress and hydric pine flatwood. In the first methane emissions study, non-steady-state rigid chambers were deployed at each community sequentially at three different times of the day during a 24- month period. Methane fluxes from the different communities did not show a discernible daily pattern, in contrast to a marked increase in seasonal emissions during inundation. All communities acted at times as temporary sinks for methane, but overall were net -2 -1 sources. Median and mean + standard error fluxes in g CH4-C.m .d were higher in the deep slough (11 and 56.2 + 22.1), followed by the wet prairie (9.01 and 53.3 + 26.6), bald cypress (3.31 and 5.54 + 2.51) and pond cypress (1.49, 4.55 + 3.35) communities. -

SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
SHELLS IN ACID Adapted from NAMEPA’s An Educator’s Guide to the Marine Environment: Shells in Acid PURPOSE Students will test the strength of normal seashells versus shells that have been soaked in vinegar to simulate the weakening effect of ocean acidification. Students identify the correlation between decreasing oceanic pH (ocean acidification) and the weakening of shells and discuss the effect this could have on the health of shellfish in the world’s oceans. MATERIALS (PER GROUP OF 4) • *white vinegar • *small, thin seashells • *non-reactive containers (glass beakers, Pyrex, measuring glass) • *water • heavy books (several) • paper towels For #6: • shells (1 per student) • snack size plastic bags (1 per student) • Small amount of vinegar • magnifying glass *Before beginning this activity, shells should be pre-soaked overnight in a 1:1 solution of vinegar and fresh water. PROCEDURE 1. Engage/Elicit Ask the students to give examples of different species of shellfish. Answers may include clams, oysters, mussels, scallops, etc. Ask students why and where they have seen these creatures. Students’ knowledge may come from eating seafood, or perhaps from having seen them in an aquarium, a marina or in coastal areas. Ask the students why these animals are important to the marine environment and to human beings. 2. Explore Lay out an assemblage of the non-soaked shells. Have the students observe the shells. Allow the students to handle the shells and ask them why the development of shells is advantageous to such animals. Explain that shellfish are invertebrates, meaning that instead of having an internal skeleton like humans, invertebrates produce a hard, protective covering. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Determining the Fraction of Organic Carbon RISC Nondefault Option
Indiana Department of Environmental Management Office of Land Quality 100 N. Senate Indianapolis, IN 46204-2251 GUIDANCE OLQ PH: (317) 232-8941 Indiana Department of Environmental Management Office of Land Quality Determining the Fraction of Organic Carbon RISC Nondefault Option Background Soil can be a complex mixture of mineral-derived compounds and organic matter. The ratios of each component can vary widely depending on the type of soil being investigated. Soil organic matter is a term used by agronomists for the total organic portion of the soil and is derived from decomposed plant matter, microorganisms, and animal residues. The decomposition process can create complex high molecular weight biopolymers (e.g., humic acid) as well as simpler organic compounds (decomposed lignin or cellulose). Only the simpler organic compounds contribute to the fraction of organic carbon. There is not a rigorous definition of the fraction of organic carbon (Foc). However, it can be thought of as the portion of the organic matter that is available to adsorb the organic contaminants of concern. The higher the organic carbon content, the more organic chemicals may be adsorbed to the soil and the less of those chemicals will be available to leach to the ground water. A nondefault option in the Risk Integrated System of Closure (RISC) is to use Foc in the Soil to Ground Water Partitioning Model to calculate a site specific migration to ground water closure level. In the Soil to Ground Water Partition Model, the coefficient, Kd, for organic compounds is the Foc multiplied by the chemical-specific soil organic carbon water partition coefficient, Koc. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -
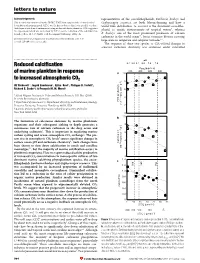
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics
CHAPTER SEVEN Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics Frans J. JorissenÃ, Christophe Fontanier and Ellen Thomas Contents 1. Introduction 263 1.1. General introduction 263 1.2. Historical overview of the use of benthic foraminiferal assemblages 266 1.3. Recent advances in benthic foraminiferal ecology 267 2. Benthic Foraminiferal Proxies: A State of the Art 271 2.1. Overview of proxy methods based on benthic foraminiferal assemblage data 271 2.2. Proxies of bottom water oxygenation 273 2.3. Paleoproductivity proxies 285 2.4. The water mass concept 301 2.5. Benthic foraminiferal faunas as indicators of current velocity 304 3. Conclusions 306 Acknowledgements 308 4. Appendix 1 308 References 313 1. Introduction 1.1. General Introduction The most popular proxies based on microfossil assemblage data produce a quanti- tative estimate of a physico-chemical target parameter, usually by applying a transfer function, calibrated on the basis of a large dataset of recent or core-top samples. Examples are planktonic foraminiferal estimates of sea surface temperature (Imbrie & Kipp, 1971) and reconstructions of sea ice coverage based on radiolarian (Lozano & Hays, 1976) or diatom assemblages (Crosta, Pichon, & Burckle, 1998). These à Corresponding author. Developments in Marine Geology, Volume 1 r 2007 Elsevier B.V. ISSN 1572-5480, DOI 10.1016/S1572-5480(07)01012-3 All rights reserved. 263 264 Frans J. Jorissen et al. methods are easy to use, apply empirical relationships that do not require a precise knowledge of the ecology of the organisms, and produce quantitative estimates that can be directly applied to reconstruct paleo-environments, and to test and tune global climate models. -

Soares, R. Et Al
Original Article DOI: 10.7860/JCDR/2017/23594.9758 Assessment of Enamel Remineralisation After Treatment with Four Different Dentistry Section Remineralising Agents: A Scanning Electron Microscopy (SEM) Study RENITA SOARES1, IDA DE NORONHA DE ATAIDE2, MARINA FERNANDES3, RAJAN LAMBOR4 ABSTRACT these groups were remineralised using the four remineralising Introduction: Decades of research has helped to increase our agents. The treated groups were subjected to pH cycling over a knowledge of dental caries and reduce its prevalence. However, period of 30 days. This was followed by assessment of surface according to World Oral Health report, dental caries still remains microhardness and SEM for qualitative evaluation of surface a major dental disease. Fluoride therapy has been utilised in changes. The results were analysed by One-Way Analysis Of a big way to halt caries progression, but has been met with Variance (ANOVA). Multiple comparisons between groups were limitations. This has paved the way for the development of performed by paired t-test and post-hoc Tukey test. newer preventive agents that can function as an adjunct to Results: The results of the study revealed that remineralisation of fluoride or independent of it. enamel was the highest in samples of Group E (Self assembling Aim: The purpose of the present study was to evaluate the ability peptide P11-4) followed by Group B (CPP-ACPF), Group C (BAG) of Casein Phosphopeptide-Amorphous Calcium Phosphate and Group D (fluoride enhanced HA gel). There was a significant Fluoride (CPP ACPF), Bioactive Glass (BAG), fluoride enhanced difference (p<0.05) in the remineralising ability between the self assembling peptide P -4 group and BAG and fluoride Hydroxyapatite (HA) gel and self-assembling peptide P11-4 to 11 remineralise artificial carious lesions in enamel in vitro using enhanced HA gel group.