SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -
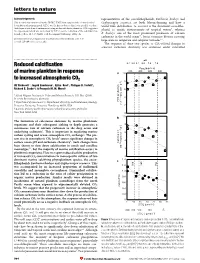
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Facies, Phosphate, and Fossil Preservation Potential Across a Lower Cambrian Carbonate Shelf, Arrowie Basin, South Australia
Palaeogeography, Palaeoclimatology, Palaeoecology 533 (2019) 109200 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Facies, phosphate, and fossil preservation potential across a Lower Cambrian T carbonate shelf, Arrowie Basin, South Australia ⁎ Sarah M. Jacqueta,b, , Marissa J. Bettsc,d, John Warren Huntleya, Glenn A. Brockb,d a Department of Geological Sciences, University of Missouri, Columbia, MO 65211, USA b Department of Biological Sciences, Macquarie University, Sydney, New South Wales 2109, Australia c Palaeoscience Research Centre, School of Environmental and Rural Science, University of New England, Armidale, New South Wales 2351, Australia d Early Life Institute and Department of Geology, State Key Laboratory for Continental Dynamics, Northwest University, Xi'an 710069, China ARTICLE INFO ABSTRACT Keywords: The efects of sedimentological, depositional and taphonomic processes on preservation potential of Cambrian Microfacies small shelly fossils (SSF) have important implications for their utility in biostratigraphy and high-resolution Calcareous correlation. To investigate the efects of these processes on fossil occurrence, detailed microfacies analysis, Organophosphatic biostratigraphic data, and multivariate analyses are integrated from an exemplar stratigraphic section Taphonomy intersecting a suite of lower Cambrian carbonate palaeoenvironments in the northern Flinders Ranges, South Biominerals Australia. The succession deepens upsection, across a low-gradient shallow-marine shelf. Six depositional Facies Hardgrounds Sequences are identifed ranging from protected (FS1) and open (FS2) shelf/lagoonal systems, high-energy inner ramp shoal complex (FS3), mid-shelf (FS4), mid- to outer-shelf (FS5) and outer-shelf (FS6) environments. Non-metric multi-dimensional scaling ordination and two-way cluster analysis reveal an underlying bathymetric gradient as the main control on the distribution of SSFs. -

Understanding the Ocean's Biological Carbon Pump in the Past: Do We Have the Right Tools?
Manuscript prepared for Earth-Science Reviews Date: 3 March 2017 Understanding the ocean’s biological carbon pump in the past: Do we have the right tools? Dominik Hülse1, Sandra Arndt1, Jamie D. Wilson1, Guy Munhoven2, and Andy Ridgwell1, 3 1School of Geographical Sciences, University of Bristol, Clifton, Bristol BS8 1SS, UK 2Institute of Astrophysics and Geophysics, University of Liège, B-4000 Liège, Belgium 3Department of Earth Sciences, University of California, Riverside, CA 92521, USA Correspondence to: D. Hülse ([email protected]) Keywords: Biological carbon pump; Earth system models; Ocean biogeochemistry; Marine sedi- ments; Paleoceanography Abstract. The ocean is the biggest carbon reservoir in the surficial carbon cycle and, thus, plays a crucial role in regulating atmospheric CO2 concentrations. Arguably, the most important single com- 5 ponent of the oceanic carbon cycle is the biologically driven sequestration of carbon in both organic and inorganic form- the so-called biological carbon pump. Over the geological past, the intensity of the biological carbon pump has experienced important variability linked to extreme climate events and perturbations of the global carbon cycle. Over the past decades, significant progress has been made in understanding the complex process interplay that controls the intensity of the biological 10 carbon pump. In addition, a number of different paleoclimate modelling tools have been developed and applied to quantitatively explore the biological carbon pump during past climate perturbations and its possible feedbacks on the evolution of the global climate over geological timescales. Here we provide the first, comprehensive overview of the description of the biological carbon pumpin these paleoclimate models with the aim of critically evaluating their ability to represent past marine 15 carbon cycle dynamics. -

Decoding the Fossil Record of Early Lophophorates
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1284 Decoding the fossil record of early lophophorates Systematics and phylogeny of problematic Cambrian Lophotrochozoa AODHÁN D. BUTLER ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9327-1 UPPSALA urn:nbn:se:uu:diva-261907 2015 Dissertation presented at Uppsala University to be publicly examined in Hambergsalen, Geocentrum, Villavägen 16, Uppsala, Friday, 23 October 2015 at 13:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Maggie Cusack (School of Geographical and Earth Sciences, University of Glasgow). Abstract Butler, A. D. 2015. Decoding the fossil record of early lophophorates. Systematics and phylogeny of problematic Cambrian Lophotrochozoa. (De tidigaste fossila lofoforaterna. Problematiska kambriska lofotrochozoers systematik och fylogeni). Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1284. 65 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9327-1. The evolutionary origins of animal phyla are intimately linked with the Cambrian explosion, a period of radical ecological and evolutionary innovation that begins approximately 540 Mya and continues for some 20 million years, during which most major animal groups appear. Lophotrochozoa, a major group of protostome animals that includes molluscs, annelids and brachiopods, represent a significant component of the oldest known fossil records of biomineralised animals, as disclosed by the enigmatic ‘small shelly fossil’ faunas of the early Cambrian. Determining the affinities of these scleritome taxa is highly informative for examining Cambrian evolutionary patterns, since many are supposed stem- group Lophotrochozoa. The main focus of this thesis pertained to the stem-group of the Brachiopoda, a highly diverse and important clade of suspension feeding animals in the Palaeozoic era, which are still extant but with only with a fraction of past diversity. -

New Finds of Skeletal Fossils in the Terminal Neoproterozoic of the Siberian Platform and Spain
New finds of skeletal fossils in the terminal Neoproterozoic of the Siberian Platform and Spain ANDREY YU. ZHURAVLEV, ELADIO LIÑÁN, JOSÉ ANTONIO GÁMEZ VINTANED, FRANÇOISE DEBRENNE, and ALEKSANDR B. FEDOROV Zhuravlev, A.Yu., Liñán, E., Gámez Vintaned, J.A., Debrenne, F., and Fedorov, A.B. 2012. New finds of skeletal fossils in the terminal Neoproterozoic of the Siberian Platform and Spain. Acta Palaeontologica Polonica 57 (1): 205–224. A current paradigm accepts the presence of weakly biomineralized animals only, barely above a low metazoan grade of or− ganization in the terminal Neoproterozoic (Ediacaran), and a later, early Cambrian burst of well skeletonized animals. Here we report new assemblages of primarily calcareous shelly fossils from upper Ediacaran (553–542 Ma) carbonates of Spain and Russia (Siberian Platform). The problematic organism Cloudina is found in the Yudoma Group of the southeastern Si− berian Platform and different skeletal taxa have been discovered in the terminal Neoproterozoic of several provinces of Spain. New data on the morphology and microstructure of Ediacaran skeletal fossils Cloudina and Namacalathus indicate that the Neoproterozoic skeletal organisms were already reasonably advanced. In total, at least 15 skeletal metazoan genera are recorded worldwide within this interval. This number is comparable with that known for the basal early Cambrian. These data reveal that the terminal Neoproterozoic skeletal bloom was a real precursor of the Cambrian radiation. Cloudina,the oldest animal with a mineralised skeleton on the Siberian Platform, characterises the uppermost Ediacaran strata of the Ust’−Yudoma Formation. While in Siberia Cloudina co−occurs with small skeletal fossils of Cambrian aspect, in Spain Cloudina−bearing carbonates and other Ediacaran skeletal fossils alternate with strata containing rich terminal Neoprotero− zoic trace fossil assemblages. -

Stratigraphic, Microfossil, and Geochemical Analysis of the Neoproterozoic Uinta Mountain Group, Utah: Evidence for a Eutrophication Event?
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2011 Stratigraphic, Microfossil, and Geochemical Analysis of the Neoproterozoic Uinta Mountain Group, Utah: Evidence for a Eutrophication Event? Dawn Schmidli Hayes Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Geology Commons, and the Sedimentology Commons Recommended Citation Hayes, Dawn Schmidli, "Stratigraphic, Microfossil, and Geochemical Analysis of the Neoproterozoic Uinta Mountain Group, Utah: Evidence for a Eutrophication Event?" (2011). All Graduate Theses and Dissertations. 874. https://digitalcommons.usu.edu/etd/874 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. STRATIGRAPHIC, MICROFOSSIL, AND GEOCHEMICAL ANALYSIS OF THE NEOPROTEROZOIC UINTA MOUNTAIN GROUP, UTAH: EVIDENCE FOR A EUTROPHICATION EVENT? by Dawn Schmidli Hayes A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Geology Approved: ______________________________ ______________________________ Dr. Carol M. Dehler Dr. John Shervais Major Advisor Committee Member ______________________________ __________________________ Dr. W. David Liddell Dr. Byron R. Burnham Committee Member Dean of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2010 ii ABSTRACT Stratigraphic, Microfossil, and Geochemical Analysis of the Neoproterozoic Uinta Mountain Group, Utah: Evidence for a Eutrophication Event? by Dawn Schmidli Hayes, Master of Science Utah State University, 2010 Major Professor: Dr. Carol M. Dehler Department: Geology Several previous Neoproterozoic microfossil diversity studies yield evidence for a relatively sudden biotic change prior to the first well‐constrained Sturtian glaciations. -

Sedimentology and Palaeontology of the Withycombe Farm Borehole, Oxfordshire, UK
Sedimentology and Palaeontology of the Withycombe Farm Borehole, Oxfordshire, England By © Kendra Morgan Power, B.Sc. (Hons.) A thesis submitted to the School of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Earth Sciences Memorial University of Newfoundland May 2020 St. John’s Newfoundland Abstract The pre-trilobitic lower Cambrian of the Withycombe Formation is a 194 m thick siliciclastic succession dominated by interbedded offshore red to purple and green pyritic mudstone with minor sandstone. The mudstone contains a hyolith-dominated small shelly fauna including: orthothecid hyoliths, hyolithid hyoliths, the rostroconch Watsonella crosbyi, early brachiopods, the foraminiferan Platysolenites antiquissimus, the coiled gastropod-like Aldanella attleborensis, halkieriids, gastropods and a low diversity ichnofauna including evidence of predation by a vagile infaunal predator. The assemblage contains a number of important index fossils (Watsonella, Platysolenites, Aldanella and the trace fossil Teichichnus) that enable correlation of strata around the base of Cambrian Stage 2 from Avalonia to Baltica, as well as the assessment of the stratigraphy within the context of the lower Cambrian stratigraphic standards of southeastern Newfoundland. The pyritized nature of the assemblage has enabled the study of some of the biota using micro-CT, augmented with petrographic studies, revealing pyritized microbial filaments of probable giant sulfur bacteria. We aim to produce the first complete description of the core and the abundant small pyritized fossils preserved in it, and develop a taphonomic model for the pyritization of the “small” shelly fossils. i Acknowledgements It is important to acknowledge and thank the many people who supported me and contributed to the successful completion of this thesis. -

Biomineralization of Plastic Waste to Improve the Strength of Plastic-Reinforced Cement Mortar
materials Article Biomineralization of Plastic Waste to Improve the Strength of Plastic-Reinforced Cement Mortar Seth Kane 1,2,* , Abby Thane 2 , Michael Espinal 1,2 , Kendra Lunday 3, Hakan Arma˘gan 4, Adrienne Phillips 2,5 , Chelsea Heveran 1,2 and Cecily Ryan 1,2 1 Mechanical and Industrial Engineering Department, Montana State University, Bozeman, MT 59717, USA; [email protected] (M.E.); [email protected] (C.H.); [email protected] (C.R.) 2 Center for Biofilm Engineering, Montana State University, Bozeman, MT 59717, USA; [email protected] (A.T.); [email protected] (A.P.) 3 Capital High School, Helena, MT 59601, USA; [email protected] 4 Omaha Burke High School, Omaha, NE 68154, USA; [email protected] 5 Civil Engineering Department, Montana State University, Bozeman, MT 59717, USA * Correspondence: [email protected]; Tel.: +1-907-750-6364 Abstract: The development of methods to reuse large volumes of plastic waste is essential to curb the environmental impact of plastic pollution. Plastic-reinforced cementitious materials (PRCs), such as plastic-reinforced mortar (PRM), may be potential avenues to productively use large quantities of low-value plastic waste. However, poor bonding between the plastic and cement matrix reduces the strength of PRCs, limiting its viable applications. In this study, calcium carbonate biomineral- ization techniques were applied to coat plastic waste and improved the compressive strength of PRM. Two biomineralization treatments were examined: enzymatically induced calcium carbon- Citation: Kane, S.; Thane, A.; ate precipitation (EICP) and microbially induced calcium carbonate precipitation (MICP). MICP Espinal, M.; Lunday, K.; Arma˘gan,H.; treatment of polyethylene terephthalate (PET) resulted in PRMs with compressive strengths similar Phillips, A.; Heveran, C.; Ryan, C. -

Volume 26C-Nogrid
Priscum Volume 26 | Issue 1 May 2021 The Newsletter of the Paleontological Society Inside this issue Diversity, Equity, and Inclusion Matter in Diversity, Equity, & Inclusion matter in Paleontology Paleontology PS Development Developments Building an inclusive and equitable Where are we now? PaleoConnect Paleontological Society (see Section 12 of the Member Code of Conduct for definitions) is Since the Paleontological Society (PS) was Journal Corner essential to realizing our core purpose — founded in 1908, its membership has been advancing the field of paleontology (see Article dominated by white men from the United PS-AGI Summer 2020 Interns II of the Articles of Incorporation). However, like States. Racial and ethnic diversity in the PS many other scientific societies, ours has remain extremely low. More than 88% of Tribute to William Clemens, Jr. historically only fostered a sense of belonging respondents to PS membership surveys Educational Materials for a subset of individuals. conducted in 2013 and 2019 self-identified as White (Stigall, 2013; unpublished data, 2019). PS Ethics Committee Report Consider your outreach experiences. Imagine These surveys revealed that, unlike the visiting a series of first grade classrooms — proportion of women, which has increased in Research and Grant Awardees overwhelmingly, the children are fascinated by younger age cohorts (Stigall, 2013), racial and PS Annual meeting at GSA Connects dinosaur bones, scale trees, and trilobites — ethnic diversity varied little among age groups, 2021 regardless of their identities. Now, reflect on suggesting that substantial barriers to the your experiences in paleontological settings as inclusion of most racial and ethnic groups have Upcoming Opportunities an adult; do they include as much diversity as persisted across generations of PS members. -

Size-Dependent Response of Foraminiferal Calcification To
Biogeosciences, 14, 3287–3308, 2017 https://doi.org/10.5194/bg-14-3287-2017 © Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Size-dependent response of foraminiferal calcification to seawater carbonate chemistry Michael J. Henehan1, David Evans1,2, Madison Shankle1, Janet E. Burke1, Gavin L. Foster3, Eleni Anagnostou3, Thomas B. Chalk3, Joseph A. Stewart3,4, Claudia H. S. Alt3,5, Joseph Durrant3, and Pincelli M. Hull1 1Department of Geology and Geophysics, Yale University, 210 Whitney Avenue, New Haven, CT 06511, USA 2School of Earth and Environmental Sciences, University of St Andrews, Irvine Building, North Street, St Andrews, Fife, KY16 9AL, UK 3Ocean and Earth Science, University of Southampton, National Oceanography Centre Southampton, Southampton, SO14 3ZH, UK 4National Institute of Standards and Technology, Hollings Marine Laboratory, 331 Ft. Johnson Road Charleston, SC 29412, USA 5Department of Biology, College of Charleston, Charleston, SC 29424, USA Correspondence to: Michael J. Henehan ([email protected]) and David Evans ([email protected]) Received: 26 October 2016 – Discussion started: 8 November 2016 Revised: 16 May 2017 – Accepted: 19 May 2017 – Published: 10 July 2017 Abstract. The response of the marine carbon cycle to of foraminiferal calcification to future change in atmospheric changes in atmospheric CO2 concentrations will be de- pCO2. termined, in part, by the relative response of calcifying and non-calcifying organisms to global change. Planktonic foraminifera are responsible for a quarter or more of global carbonate production, therefore understanding the sensitivity of calcification in these organisms to environmental change 1 Introduction is critical. Despite this, there remains little consensus as to whether, or to what extent, chemical and physical factors af- Calcium carbonate (CaCO3) production and transport to the fect foraminiferal calcification.