Protist Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antony Van Leeuwenhoek, the Father of Microscope
Turkish Journal of Biochemistry – Türk Biyokimya Dergisi 2016; 41(1): 58–62 Education Sector Letter to the Editor – 93585 Emine Elif Vatanoğlu-Lutz*, Ahmet Doğan Ataman Medicine in philately: Antony Van Leeuwenhoek, the father of microscope Pullardaki tıp: Antony Van Leeuwenhoek, mikroskobun kaşifi DOI 10.1515/tjb-2016-0010 only one lens to look at blood, insects and many other Received September 16, 2015; accepted December 1, 2015 objects. He was first to describe cells and bacteria, seen through his very small microscopes with, for his time, The origin of the word microscope comes from two Greek extremely good lenses (Figure 1) [3]. words, “uikpos,” small and “okottew,” view. It has been After van Leeuwenhoek’s contribution,there were big known for over 2000 years that glass bends light. In the steps in the world of microscopes. Several technical inno- 2nd century BC, Claudius Ptolemy described a stick appear- vations made microscopes better and easier to handle, ing to bend in a pool of water, and accurately recorded the which led to microscopy becoming more and more popular angles to within half a degree. He then very accurately among scientists. An important discovery was that lenses calculated the refraction constant of water. During the combining two types of glass could reduce the chromatic 1st century,around year 100, glass had been invented and effect, with its disturbing halos resulting from differences the Romans were looking through the glass and testing in refraction of light (Figure 2) [4]. it. They experimented with different shapes of clear glass In 1830, Joseph Jackson Lister reduced the problem and one of their samples was thick in the middle and thin with spherical aberration by showing that several weak on the edges [1]. -

SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
SHELLS IN ACID Adapted from NAMEPA’s An Educator’s Guide to the Marine Environment: Shells in Acid PURPOSE Students will test the strength of normal seashells versus shells that have been soaked in vinegar to simulate the weakening effect of ocean acidification. Students identify the correlation between decreasing oceanic pH (ocean acidification) and the weakening of shells and discuss the effect this could have on the health of shellfish in the world’s oceans. MATERIALS (PER GROUP OF 4) • *white vinegar • *small, thin seashells • *non-reactive containers (glass beakers, Pyrex, measuring glass) • *water • heavy books (several) • paper towels For #6: • shells (1 per student) • snack size plastic bags (1 per student) • Small amount of vinegar • magnifying glass *Before beginning this activity, shells should be pre-soaked overnight in a 1:1 solution of vinegar and fresh water. PROCEDURE 1. Engage/Elicit Ask the students to give examples of different species of shellfish. Answers may include clams, oysters, mussels, scallops, etc. Ask students why and where they have seen these creatures. Students’ knowledge may come from eating seafood, or perhaps from having seen them in an aquarium, a marina or in coastal areas. Ask the students why these animals are important to the marine environment and to human beings. 2. Explore Lay out an assemblage of the non-soaked shells. Have the students observe the shells. Allow the students to handle the shells and ask them why the development of shells is advantageous to such animals. Explain that shellfish are invertebrates, meaning that instead of having an internal skeleton like humans, invertebrates produce a hard, protective covering. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -
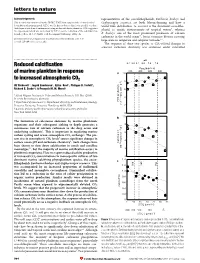
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

8113-Yasham Neden Var-Nick Lane-Ebru Qilic-2015-318S.Pdf
KOÇ ÜNiVERSiTESi YAYINLARI: 87 BiYOLOJi Yaşam Neden Var? Nick Lane lngilizceden çeviren: Ebru Kılıç Yayına hazırlayan: Hülya Haripoğlu Düzelti: Elvan Özkaya iç rasarım: Kamuran Ok Kapak rasarımı: James Jones The Vital Question © Nick Lane, 2015 ©Koç Üniversiresi Yayınları, 2015 1. Baskı: lsranbul, Nisan 2016 Bu kitabın yazarı, eserin kendi orijinal yararımı olduğunu ve eserde dile getirilen rüm görüşlerin kendisine air olduğunu, bunlardan dolayı kendisinden başka kimsenin sorumlu rurulamayacağını; eserde üçüncü şahısların haklarını ihlal edebilecek kısımlar olmadığını kabul eder. Baskı: 12.marbaa Sertifika no: 33094 Naro Caddesi 14/1 Seyranrepe Kağırhane/lsranbul +90 212 284 0226 Koç Üniversiresi Yayınları lsriklal Caddesi No:181 Merkez Han Beyoğlu/lsranbul +90 212 393 6000 [email protected] • www.kocuniversirypress.com • www.kocuniversiresiyayinlari.com Koç Universiry Suna Kıraç Library Caraloging-in-Publicarion Dara Lane, Nick, 1967- Yaşam neden var?/ Nick Lane; lngilizceden çeviren Ebru Kılıç; yayına hazırlayan Hülya Haripoğlu. pages; cm. lncludes bibliographical references and index. ISBN 978-605-5250-94-2 ı. Life--Origin--Popular works. 2. Cells. 1. Kılıç, Ebru. il. Haripoğlu, Hülya. 111. Tirle. QH325.L3520 2016 Yaşam Neden Var? NICKLANE lngilizceden Çeviren: Ebru Kılıç ffi1KÜY İçindeki le� Resim Listesi 7 TEŞEKKÜR 11 GiRİŞ 17 Yaşam Neden Olduğu Gibidir? BiRİNCi BÖLÜM 31 Yaşam Nedir? Yaşamın ilk 2 Milyar Yılının Kısa Ta rihi 35 Genler ve Doğal Ortamla ilgili Sorun 39 Biyolojinin Kalbindeki Kara Delik 43 Karmaşıklık Yo lunda Kayıp Adımlar -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Protists/Fungi Station Lab Information
Protists/Fungi Station Lab Information 1 Protists Information Background: Perhaps the most strikingly diverse group of organisms on Earth is that of the Protists, Found almost anywhere there is water – from puddles to sediments. Protists rely on water. Somea re marine (salt water), some are freshwater, some are terrestrial (land dwellers) in moist soil and some are parasites which live in the tissues of others. The Protist kingdom is made up of a wide variety of eukaryotic cells. All protist cells have nuclei and other characteristics eukaryotic features. Some protists have more than one nucleus and are called “multinucleated”. Cellular Organization: Protists show a variety in cellular organization: single celled (unicellular), groups of single cells living together in a close and permanent association (colonies or filaments) or many cells = multicellular organization (ex. Seaweed). Obtaining food: There is a variety in how protists get their food. Like plants, many protists are autotrophs, meaning they make their own food through photosynthesis and store it as starch. It is estimated that green protist cells chemically capture and process over a billion tons of carbon in the Earth’s oceans and freshwater ponds every year. Photosynthetic or “green” protists have a multitude of membrane-enclosed bags (chloroplasts) which contain the photosynthetic green pigment called chlorophyll. Many of these organisms’ cell walls are similar to that of plant cells and are made of cellulose. Others are “heterotrophs”. Like animals, they eat other organisms or, like fungi, receiving their nourishment from absorbing nutrient molecules from their surroundings or digest living things. Some are parasitic and feed off of a living host. -

Facies, Phosphate, and Fossil Preservation Potential Across a Lower Cambrian Carbonate Shelf, Arrowie Basin, South Australia
Palaeogeography, Palaeoclimatology, Palaeoecology 533 (2019) 109200 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Facies, phosphate, and fossil preservation potential across a Lower Cambrian T carbonate shelf, Arrowie Basin, South Australia ⁎ Sarah M. Jacqueta,b, , Marissa J. Bettsc,d, John Warren Huntleya, Glenn A. Brockb,d a Department of Geological Sciences, University of Missouri, Columbia, MO 65211, USA b Department of Biological Sciences, Macquarie University, Sydney, New South Wales 2109, Australia c Palaeoscience Research Centre, School of Environmental and Rural Science, University of New England, Armidale, New South Wales 2351, Australia d Early Life Institute and Department of Geology, State Key Laboratory for Continental Dynamics, Northwest University, Xi'an 710069, China ARTICLE INFO ABSTRACT Keywords: The efects of sedimentological, depositional and taphonomic processes on preservation potential of Cambrian Microfacies small shelly fossils (SSF) have important implications for their utility in biostratigraphy and high-resolution Calcareous correlation. To investigate the efects of these processes on fossil occurrence, detailed microfacies analysis, Organophosphatic biostratigraphic data, and multivariate analyses are integrated from an exemplar stratigraphic section Taphonomy intersecting a suite of lower Cambrian carbonate palaeoenvironments in the northern Flinders Ranges, South Biominerals Australia. The succession deepens upsection, across a low-gradient shallow-marine shelf. Six depositional Facies Hardgrounds Sequences are identifed ranging from protected (FS1) and open (FS2) shelf/lagoonal systems, high-energy inner ramp shoal complex (FS3), mid-shelf (FS4), mid- to outer-shelf (FS5) and outer-shelf (FS6) environments. Non-metric multi-dimensional scaling ordination and two-way cluster analysis reveal an underlying bathymetric gradient as the main control on the distribution of SSFs. -

Brown Algae and 4) the Oomycetes (Water Molds)
Protista Classification Excavata The kingdom Protista (in the five kingdom system) contains mostly unicellular eukaryotes. This taxonomic grouping is polyphyletic and based only Alveolates on cellular structure and life styles not on any molecular evidence. Using molecular biology and detailed comparison of cell structure, scientists are now beginning to see evolutionary SAR Stramenopila history in the protists. The ongoing changes in the protest phylogeny are rapidly changing with each new piece of evidence. The following classification suggests 4 “supergroups” within the Rhizaria original Protista kingdom and the taxonomy is still being worked out. This lab is looking at one current hypothesis shown on the right. Some of the organisms are grouped together because Archaeplastida of very strong support and others are controversial. It is important to focus on the characteristics of each clade which explains why they are grouped together. This lab will only look at the groups that Amoebozoans were once included in the Protista kingdom and the other groups (higher plants, fungi, and animals) will be Unikonta examined in future labs. Opisthokonts Protista Classification Excavata Starting with the four “Supergroups”, we will divide the rest into different levels called clades. A Clade is defined as a group of Alveolates biological taxa (as species) that includes all descendants of one common ancestor. Too simplify this process, we have included a cladogram we will be using throughout the SAR Stramenopila course. We will divide or expand parts of the cladogram to emphasize evolutionary relationships. For the protists, we will divide Rhizaria the supergroups into smaller clades assigning them artificial numbers (clade1, clade2, clade3) to establish a grouping at a specific level. -

Understanding the Ocean's Biological Carbon Pump in the Past: Do We Have the Right Tools?
Manuscript prepared for Earth-Science Reviews Date: 3 March 2017 Understanding the ocean’s biological carbon pump in the past: Do we have the right tools? Dominik Hülse1, Sandra Arndt1, Jamie D. Wilson1, Guy Munhoven2, and Andy Ridgwell1, 3 1School of Geographical Sciences, University of Bristol, Clifton, Bristol BS8 1SS, UK 2Institute of Astrophysics and Geophysics, University of Liège, B-4000 Liège, Belgium 3Department of Earth Sciences, University of California, Riverside, CA 92521, USA Correspondence to: D. Hülse ([email protected]) Keywords: Biological carbon pump; Earth system models; Ocean biogeochemistry; Marine sedi- ments; Paleoceanography Abstract. The ocean is the biggest carbon reservoir in the surficial carbon cycle and, thus, plays a crucial role in regulating atmospheric CO2 concentrations. Arguably, the most important single com- 5 ponent of the oceanic carbon cycle is the biologically driven sequestration of carbon in both organic and inorganic form- the so-called biological carbon pump. Over the geological past, the intensity of the biological carbon pump has experienced important variability linked to extreme climate events and perturbations of the global carbon cycle. Over the past decades, significant progress has been made in understanding the complex process interplay that controls the intensity of the biological 10 carbon pump. In addition, a number of different paleoclimate modelling tools have been developed and applied to quantitatively explore the biological carbon pump during past climate perturbations and its possible feedbacks on the evolution of the global climate over geological timescales. Here we provide the first, comprehensive overview of the description of the biological carbon pumpin these paleoclimate models with the aim of critically evaluating their ability to represent past marine 15 carbon cycle dynamics.