Calcification, a Physiological Process to Be Considered in the Context Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endoskeleton
THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON AN ESSAY ON THE SIGNIFICANCE AND) MEANING OF' SEGMENTATION IN COELOMATE ANIMIALS By W1T. 13. PRIMIROSE, M.B.. Cii.B. Lately Seniior Deniionistrator of lAnatomtiy i? the (nWizversity (of Glasgozc THE EVOLUTION OF THE VERTEBRATE ENDOSKELETON WVHEN investigating the morphology of the vertebrate head, I found it necessary to discover the morphological principles on which the segmentation of the body is founded. This essay is one of the results of this investigations, and its object is to show what has determined the segmented form in vertebrate animals. It will be seen that the segmented form in vertebrates results from a condition which at no time occurs in vertebrate animals. This condition is a form of skeleton found only in animals lower in the scale of organisation than vertebrates, and has the characters of a space containing water. This space is the Coelomic Cavity. The coelomic cavity is the key to the formation of the segmented structure of the body, and is the structure that determines the vertebrate forni. The coclomic cavity is present in a well defined state from the Alnelida tuwards, so that in ainielides it is performing the functions for which a coelom was evolved. It is, however, necessary to observe the conditions prevailing anon)g still lower forms to see why a separate cavity was formed in animals, which became the means of raising them in the scale of organisation, and ultimately leading to the evolution of the vertebrate animal. I therefore propose to trace the steps in evolution by which, I presume, the coelomic cavity originated, and then show how it or its modifications have been the basis on which the whole vertebrate structure of animals is founded. -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

SHELLS in ACID Adapted from NAMEPA’S an Educator’S Guide to the Marine Environment: Shells in Acid
SHELLS IN ACID Adapted from NAMEPA’s An Educator’s Guide to the Marine Environment: Shells in Acid PURPOSE Students will test the strength of normal seashells versus shells that have been soaked in vinegar to simulate the weakening effect of ocean acidification. Students identify the correlation between decreasing oceanic pH (ocean acidification) and the weakening of shells and discuss the effect this could have on the health of shellfish in the world’s oceans. MATERIALS (PER GROUP OF 4) • *white vinegar • *small, thin seashells • *non-reactive containers (glass beakers, Pyrex, measuring glass) • *water • heavy books (several) • paper towels For #6: • shells (1 per student) • snack size plastic bags (1 per student) • Small amount of vinegar • magnifying glass *Before beginning this activity, shells should be pre-soaked overnight in a 1:1 solution of vinegar and fresh water. PROCEDURE 1. Engage/Elicit Ask the students to give examples of different species of shellfish. Answers may include clams, oysters, mussels, scallops, etc. Ask students why and where they have seen these creatures. Students’ knowledge may come from eating seafood, or perhaps from having seen them in an aquarium, a marina or in coastal areas. Ask the students why these animals are important to the marine environment and to human beings. 2. Explore Lay out an assemblage of the non-soaked shells. Have the students observe the shells. Allow the students to handle the shells and ask them why the development of shells is advantageous to such animals. Explain that shellfish are invertebrates, meaning that instead of having an internal skeleton like humans, invertebrates produce a hard, protective covering. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Murphey Et Al. 2019 Best Practices in Mitigation Paleontology
PROCEEDINGS of the San Diego Society of Natural History Founded 1874 Number 47 1 May 2019 BEST PRACTICES IN MITIGATION PALEONTOLOGY By Paul C. Murphey Paleo Solutions, 2785 Speer Boulevard, Suite 1, Denver, CO 80211, U.S.A.; [email protected]; Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Department of Earth Sciences, Denver Museum of Nature and Science, 2001 Colorado Boulevard, Denver, CO 80201, U.S.A. Georgia E. Knauss SWCA Environmental Consultants, 1892 S. Sheridan Avenue, Sheridan, WY 82801 U.S.A.; [email protected] Lanny H. Fisk PaleoResource Consultants, 550 High Street, Suite 108, Auburn, CA 95603, U.S.A. (deceased) Thomas A. Deméré Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] Robert E. Reynolds Department of Paleontology, San Diego Natural History Museum, 1788 El Prado, San Diego, CA 92101, U.S.A.; [email protected] For correspondence, write to: Paul C. Murphey, Paleo Solutions, 4614 Lonespur Ct. Oceanside, CA 92056 Email: [email protected] [email protected] bpmp-19-01-fm Page 2 PDF Created: 2019-4-12: 9:20:AM 2 Paul C. Murphey, Georgia E. Knauss, Lanny H. Fisk, Thomas A. Deméré, and Robert E. Reynolds TABLE OF CONTENTS Abstract . 4 Introduction . 4 History and Scientific Contributions . 5 History of Mitigation Paleontology in the United States . 5 Methods Best Practice Categories . 7 1. Qualifications. 7 Confusion between Resource Disciplines . 7 Professional Geologists as Mitigation Paleontologists. 8 Mitigation Paleontologist Categories . -
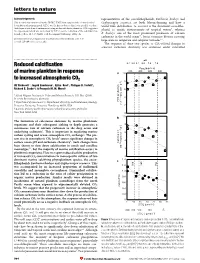
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Biology of Echinoderms
Echinoderms Branches on the Tree of Life Programs ECHINODERMS Written and photographed by David Denning and Bruce Russell Produced by BioMEDIA ASSOCIATES ©2005 - Running time 16 minutes. Order Toll Free (877) 661-5355 Order by FAX (843) 470-0237 The Phylum Echinodermata consists of about 6,000 living species, all of which are marine. This video program compares the five major classes of living echinoderms in terms of basic functional biology, evolution and ecology using living examples, animations and a few fossil species. Detailed micro- and macro- photography reveal special adaptations of echinoderms and their larval biology. (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE VIDEO PROGRAM) Summary of the Program: Introduction - Characteristics of the Class Echinoidea phylum. spine adaptations, pedicellaria, Aristotle‘s lantern, sand dollars, urchin development, Class Asteroidea gastrulation, settlement skeleton, water vascular system, tube feet function, feeding, digestion, Class Holuthuroidea spawning, larval development, diversity symmetry, water vascular system, ossicles, defensive mechanisms, diversity, ecology Class Ophiuroidea regeneration, feeding, diversity Class Crinoidea – Topics ecology, diversity, fossil echinoderms © BioMEDIA ASSOCIATES (1 of 7) Echinoderms ... ... The characteristics that distinguish Phylum Echinodermata are: radial symmetry, internal skeleton, and water-vascular system. Echinoderms appear to be quite different than other ‘advanced’ animal phyla, having radial (spokes of a wheel) symmetry as adults, rather than bilateral (worm-like) symmetry as in other triploblastic (three cell-layer) animals. Viewers of this program will observe that echinoderm radial symmetry is secondary; echinoderms begin as bilateral free-swimming larvae and become radial at the time of metamorphosis. Also, in one echinoderm group, the sea cucumbers, partial bilateral symmetry is retained in the adult stages -- sea cucumbers are somewhat worm–like. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation. -

Facies, Phosphate, and Fossil Preservation Potential Across a Lower Cambrian Carbonate Shelf, Arrowie Basin, South Australia
Palaeogeography, Palaeoclimatology, Palaeoecology 533 (2019) 109200 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Facies, phosphate, and fossil preservation potential across a Lower Cambrian T carbonate shelf, Arrowie Basin, South Australia ⁎ Sarah M. Jacqueta,b, , Marissa J. Bettsc,d, John Warren Huntleya, Glenn A. Brockb,d a Department of Geological Sciences, University of Missouri, Columbia, MO 65211, USA b Department of Biological Sciences, Macquarie University, Sydney, New South Wales 2109, Australia c Palaeoscience Research Centre, School of Environmental and Rural Science, University of New England, Armidale, New South Wales 2351, Australia d Early Life Institute and Department of Geology, State Key Laboratory for Continental Dynamics, Northwest University, Xi'an 710069, China ARTICLE INFO ABSTRACT Keywords: The efects of sedimentological, depositional and taphonomic processes on preservation potential of Cambrian Microfacies small shelly fossils (SSF) have important implications for their utility in biostratigraphy and high-resolution Calcareous correlation. To investigate the efects of these processes on fossil occurrence, detailed microfacies analysis, Organophosphatic biostratigraphic data, and multivariate analyses are integrated from an exemplar stratigraphic section Taphonomy intersecting a suite of lower Cambrian carbonate palaeoenvironments in the northern Flinders Ranges, South Biominerals Australia. The succession deepens upsection, across a low-gradient shallow-marine shelf. Six depositional Facies Hardgrounds Sequences are identifed ranging from protected (FS1) and open (FS2) shelf/lagoonal systems, high-energy inner ramp shoal complex (FS3), mid-shelf (FS4), mid- to outer-shelf (FS5) and outer-shelf (FS6) environments. Non-metric multi-dimensional scaling ordination and two-way cluster analysis reveal an underlying bathymetric gradient as the main control on the distribution of SSFs. -

Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs
Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs Coral reefs are vital to the long-term to produce CaCO3, carbon dioxide (CO2), As CO2 increases in the atmosphere, viability of coastal societies, providing and water (H2O). Over time as these more is absorbed by the surface of the economic, recreational, and aesthetic organisms grow and die, their skeletons ocean, where it combines with seawater value from which coastal communities break down and become calcium carbon- to make a weak acid called carbonic thrive. Some of the services that coral ate sediments. These sediments fill in acid (H2CO3). This process, called reefs provide include protection from the framework of the reef and eventually ocean acidification, causes a decrease storm waves, nurseries and habitats for become cemented together, construct- in seawater pH (or increase in acidity) commercially important fish species, ing the foundation for continued upward that can result in a decrease in biogenic and production of sand for beaches. growth of the reef structure. The infilling calcification rates, dissolution of carbon- Coral reefs develop over thousands of the reef framework with sediments is ate sediments, and loss of reef structure. of years as tropical marine organ- what allows vertical accretion over time One of the primary concerns associated isms build skeletons of calcium carbonate and enables reef growth to keep up with with ocean acidification is whether coral (CaCO3) minerals to form a three-dimen- sea-level rise. Calcification is a revers- reefs will be able to continue to grow at sional structure (fig. 1). This process, ible process. -

Aragonite Formation in Confinements: a Step Toward Understanding Polymorph Control COMMENTARY Yifei Xua,B,C and Nico A
COMMENTARY Aragonite formation in confinements: A step toward understanding polymorph control COMMENTARY Yifei Xua,b,c and Nico A. J. M. Sommerdijka,b,c,1 Calcium carbonate (CaCO3) is one of the most com- unclear but were generally attributed to the interac- mon minerals on Earth; it not only forms rocks like tion between the acidic functional groups of the limestone or marble but is also a main component of biomacromolecules and the mineral components. Ad- biominerals such as pearls, the nacre of seashells, and ditionally, it was reported that a macromolecular sea-urchin skeletons (1). Despite many years of re- hydrogel-like 3D network is formed before the miner- search, the polymorphism of CaCO3 is still far from alization of nacre (11), which may play a role in the being understood. CaCO3 has three anhydrous crys- crystallization process by confining the crystallization talline forms: calcite, aragonite, and vaterite, with a to defined small volumes. Nonetheless, confinement decreasing thermodynamic stability under aqueous has never been directly correlated with aragonite for- ambient conditions (calcite > aragonite > vaterite) mation in biominerals, although its capability of con- (2). While vaterite is rare in nature, calcite and arago- trolling crystal orientation and polymorphism has nite are both frequently found in rocks or biominerals been shown in many recent reports (12–15). (1). A well-known example is the aragonite structure of Now, Zeng et al. (5) explore for the first time the nacre (3), where the organization of the crystals leads impact of confinement on aragonite formation. By to extraordinary mechanical performance. However, precipitating CaCO3 within the cylindrical pores of in synthetic systems, crystallization experiments only track-etched membranes (Fig. -

ZOOLOGY Zoology 109 (2006) 164–168
ARTICLE IN PRESS ZOOLOGY Zoology 109 (2006) 164–168 www.elsevier.de/zool Mineralized Cartilage in the skeleton of chondrichthyan fishes Mason N. Dean, Adam P. Summersà Ecology and Evolutionary Biology, University of California – Irvine, 321 Steinhaus Hall, Irvine, CA 92697-2525, USA Received 10 February 2006; received in revised form 2 March 2006; accepted 3 March 2006 Abstract The cartilaginous endoskeleton of chondrichthyan fishes (sharks, rays, and chimaeras) exhibits complex arrangements and morphologies of calcified tissues that vary with age, species, feeding behavior, and location in the body. Understanding of the development, evolutionary history and function of these tissue types has been hampered by the lack of a unifying terminology. In order to facilitate reciprocal illumination between disparate fields with convergent interests, we present levels of organization in which crystal orientation/size delimits three calcification types (areolar, globular, and prismatic) that interact in two distinct skeletal types, vertebral and tessellated cartilage. The tessellated skeleton is composed of small blocks (tesserae) of calcified cartilage (both prismatic and globular) overlying a core of unmineralized cartilage, while vertebral cartilage usually contains all three types of calcification. r 2006 Elsevier GmbH. All rights reserved. Keywords: Elasmobranch skeleton; Mineralization; Calcified cartilage; Tesserae Introduction (Summers, 2000; Schaefer and Summers, 2005; Dean et al., 2006) interests in cartilaginous skeletons, we need The breadth of morphological variation of the a common language. Furthermore, the current termi- mineralized cartilage of the endoskeleton of chon- nology masks unappreciated complexity in morphology drichthyan fishes (sharks, rays, and chimaeras) has led that will fuel future research in several of these fields.