Role of Phosphate in Biomineralization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Four Hundred Million Years of Silica Biomineralization in Land Plants
Four hundred million years of silica biomineralization in land plants Elizabeth Trembath-Reicherta,1, Jonathan Paul Wilsonb, Shawn E. McGlynna,c, and Woodward W. Fischera aDivision of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125; bDepartment of Biology, Haverford College, Haverford, PA 19041; and cGraduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji-shi, Tokyo 192-0397, Japan Edited by Thure E. Cerling, University of Utah, Salt Lake City, UT, and approved February 20, 2015 (received for review January 7, 2015) Biomineralization plays a fundamental role in the global silicon Silica is widely used within plants for structural support and cycle. Grasses are known to mobilize significant quantities of Si in pathogen defense (19–21), but it remains a poorly understood the form of silica biominerals and dominate the terrestrial realm aspect of plant biology. Recent work on the angiosperm Oryza today, but they have relatively recent origins and only rose to sativa demonstrated that silica accumulation is facilitated by taxonomic and ecological prominence within the Cenozoic Era. transmembrane proteins expressed in root cells (21–24). Phy- This raises questions regarding when and how the biological silica logenetic analysis revealed that these silicon transport proteins cycle evolved. To address these questions, we examined silica were derived from a diverse family of modified aquaporins that abundances of extant members of early-diverging land plant include arsenite and glycerol transporters (19, 21, 25, 26). A clades, which show that silica biomineralization is widespread different member of this aquaporin family was recently identi- across terrestrial plant linages. Particularly high silica abundances fied that enables silica uptake in the horsetail Equisetum,an are observed in lycophytes and early-diverging ferns. -

Early Manifestation of Calcinosis Cutis in Pseudohypoparathyroidism Type
European Journal of Endocrinology (2005) 152 515–519 ISSN 0804-4643 CASE REPORT Early manifestation of calcinosis cutis in pseudohypoparathyroidism type Ia associated with a novel mutation in the GNAS gene Felix G Riepe, Wiebke Ahrens1, Nils Krone, Regina Fo¨lster-Holst2, Jochen Brasch2, Wolfgang G Sippell, Olaf Hiort1 and Carl-Joachim Partsch3 Department of Pediatrics, Division of Pediatric Endocrinology, Universita¨tsklinikum Schleswig-Holstein, Campus Kiel, Christian-Albrechts-Universita¨t, 24105 Kiel, Germany, 1Department of Pediatrics, Division of Pediatric Endocrinology and Diabetes, Universita¨tsklinikum Schleswig-Holstein, Campus Lu¨beck, Universita¨t zu Lu¨beck, 23538 Lu¨beck, Germany, 2Department of Dermatology, Universita¨tsklinikum Schleswig-Holstein, Campus Kiel, Christian-Albrechts-Universita¨t, 24105 Kiel, Germany and 3Children’s Hospital, Sta¨dtische Kliniken Esslingen, 73730 Esslingen a.N., Germany (Correspondence should be addressed to W G Sippell; Email: [email protected]) Abstract Objective: To clarify the molecular defect for the clinical finding of congenital hypothyroidism com- bined with the manifestation of calcinosis cutis in infancy. Case report: The male patient presented with moderately elevated blood thyrotropin levels at neonatal screening combined with slightly decreased plasma thyroxine and tri-iodothyronine concentrations, necessitating thyroid hormone substitution 2 weeks after birth. At the age of 7 months calcinosis cutis was seen and the patient underwent further investigation. Typical features of Albright’s heredi- tary osteodystrophy (AHO), including round face, obesity and delayed psychomotor development, were found. Methods and results: Laboratory investigation revealed a resistance to parathyroid hormone (PTH) with highly elevated PTH levels and a reduction in adenylyl cyclase-stimulating protein (Gsa) activity leading to the diagnosis of pseudohypoparathyroidism type Ia (PHP Ia). -

Sarcoidosis, Hypercalcemia and Calcium-Sensing Receptor Mutation: a Case Report
468 Letters to the Editor Sarcoidosis, Hypercalcemia and Calcium-sensing Receptor Mutation: A Case Report Shreya Dixit1 and Pablo Fernandez-Peñas1,2* 1Skin and Cancer Foundation Australia, 7 Ashley Lane, Westmead, NSW 2145, and 2Sydney Medical School Western, The University of Sydney, NSW, Australia. *E-mail: [email protected] Accepted October 28, 2010. Sarcoidosis is a multisystem granulomatous disease of The incidence of hypercalcaemia in patients with unknown aetiology. It predominantly affects the lungs; sarcoidosis is approximately 10% (3). It has previously however, 25% of patients have skin involvement, ranging been found that these abnormalities of calcium metabo- from non-specific maculopapular eruptions to erythema lism are due to dysregulated production of calcitriol by nodosum and lupus pernio (1). activated macrophages trapped in pulmonary alveoli and Familial hypocalciuric hypercalcaemia (FHH) results granulomatous inflammation (4). Conversion of calcidiol from an autosomal dominantly inherited inactivating to calcitriol is facilitated by 1 alpha-hydroxylase, a cyto- mutation of the calcium-sensing receptor (CaSR) gene chrome P450 enzyme. The activity of 1 alpha-hydroxyla- and is typically asymptomatic (2). Sarcoidosis has not se is tightly regulated through complex mechanisms that previously been reported in FHH. We had the opportu- depend on the circulating levels of calcium, phosphorus, nity to study a patient with sarcoidosis that has a CaSR PTH, 1,25 (OH)2D3 (calcitriol) and calcitonin. The role mutation. of CaSR in the regulation of 1 alpha-hydroxylase has not been defined, although there are suggestions that CaSR activation can repress the enzyme (5). CASE REPORT The incidence of FHH in patients with sarcoidosis A 70-year-old woman was referred by her endocrinologist with is unknown. -

Tumoral Calcinosis and Calciphylaxis Treated with Subtotal Parathyroidectomy and Sodium Thiosulphate
CASE REPORT eISSN 2384-0293 Yeungnam Univ J Med 2016;33(1):68-71 http://dx.doi.org/10.12701/yujm.2016.33.1.68 Tumoral calcinosis and calciphylaxis treated with subtotal parathyroidectomy and sodium thiosulphate Hyunjeong Cho1, Yongjin Yi1, Eunjeong Kang1, Seokwoo Park1, Eun Jin Cho2, Sung Tae Cho3, Rho Won Chun3, Kyu Eun Lee4, Kook-Hwan Oh1 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul; 2Department of Internal Medicine, Hongseong Medical Center, Hongseong; 3Dr. Chun & Cho`s Medical Clinic & Dialysis Center; 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea Tumoral calcinosis (TC) is a condition resulting from extensive calcium phosphate precipitation, primarily in the periarticular tissues around major joints. Calciphylaxis is a fatal ischemic vasculopathy mainly affecting dermal blood vessels and subcutaneous fat. This syndrome is rare and predominantly occurs in patients with end-stage renal disease. Here, we report on a rare case involving a patient with TC complicated with calciphylaxis. Our patient was a 31-year-old man undergoing hemodialysis who presented with masses on both shoulders and necrotic cutaneous ulcers, which were associated with secondary hyperparathyroidism, on his lower legs. He underwent subtotal parathyroidectomy, and sodium thiosulfate (STS) was administered for 27 weeks. Twenty months after beginning the STS treatment course, he experienced dramatic relief of his TC and calciphylaxis. Keywords: Tumoral calcinosis; Calciphylaxis; Parathyroidectomy; Sodium thiosulphate INTRODUCTION reported to range from 0.5-3% [2]. The pathomechanisms of these two disorders are poorly understood, but believed to Calcinosis cutis is characterized by the deposition of in- be related to disturbances in mineral metabolism. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
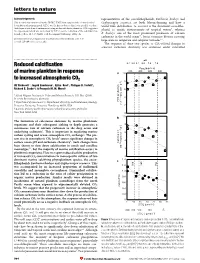
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Section XI Extraskeletal (Ectopic) Calcification and Ossification
Section XI Extraskeletal (Ectopic) Calcification and Ossification Michael P. Whyte Division of Bone and Mineral Diseases, Washington University School of Medicine at Barnes-Jewish Hospital and Center for Metabolic Bone Disease and Molecular Research, Shriners Hospitals for Children, St. Louis, Missouri INTRODUCTION somewhat higher value because they have greater serum phos- phate concentrations compared with adults. However, this is A significant number and variety of disorders cause extraskel- not well established.(5) etal deposition of calcium and phosphate (Table 1). In some, The material that comprises metastatic calcification may be mineral is precipitated as amorphous calcium phosphate or as amorphous calcium phosphate initially, but hydroxyapatite is crystals of hydroxyapatite; in others, osseous tissue is formed. deposited soon after.(2) The anatomic pattern of deposition The pathogenesis of ectopic mineralization is generally attrib- varies somewhat between hypercalcemia and hyperphos- uted to one of three mechanisms (Table 1). First, a supranormal phatemia, but occurs irrespective of the specific underlying “calcium-phosphate solubility product” in extracellular fluid condition or mechanism for the disturbed mineral homeostasis. can cause metastatic calcification. Second, mineral may be Additionally, there is a predilection for certain tissues. deposited as dystrophic calcification into metabolically im- Hypercalcemia is typically associated with mineral deposits paired or dead tissue despite normal serum levels of calcium in the kidneys, lungs, and fundus of the stomach. In these and phosphate. Third, ectopic ossification (or true bone forma- “acid-secreting” organs, a local alkaline milieu may account tion) occurs in a few disorders for which the pathogenesis is for the calcium deposition. In addition, the media of large becoming increasingly understood. -

Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O
1122 Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O. Box 80021 3508 TA Utrecht, The Netherlands INTRODUCTION Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere- hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment. Biogeochemical cycles provide a general framework in which geochemists organize their knowledge and interpret their data. The cycle of a given element or substance maps out the rates of transformation in, and transport fluxes between, adjoining environmental reservoirs. The temporal and spatial scales of interest dictate the selection of reservoirs and processes included in the cycle. Typically, the need for a detailed representation of biological process rates and ecosystem structure decreases as the spatial and temporal time scales considered increase. Much progress has been made in the development of global-scale models of biogeochemical cycles. Although these models are based on fairly simple representations of the biosphere and hydrosphere, they account for the large-scale changes in the composition, redox state and biological productivity of the earth surface environment that have occurred over geological time. -

Distribution of Calcium Phosphate in the Exoskeleton of Larval Exeretonevra Angustifrons Hardy (Diptera: Xylophagidae)
Arthropod Structure & Development 34 (2005) 41–48 www.elsevier.com/locate/asd Distribution of calcium phosphate in the exoskeleton of larval Exeretonevra angustifrons Hardy (Diptera: Xylophagidae) Bronwen W. Cribba,b,*, Ron Rascha, John Barrya, Christopher M. Palmerb,1 aCentre for Microscopy and Microanalysis, The University of Queensland, Brisbane, Qd 4072, Australia bDepartment of Zoology and Entomology, The University of Queensland, Brisbane, Qd 4072, Australia Received 28 July 2004; accepted 26 August 2004 Abstract Distribution and organisation of the mineral, amorphous calcium phosphate (ACP), has been investigated in the exoskeleton of the xylophagid fly larva Exeretonevra angustifrons Hardy. While head capsule and anal plate are smooth with a thin epicuticle, the epicuticle of the body is thicker and shows unusual micro-architecture comprised of minute hemispherical (dome-shaped) protrusions. Electron microprobe analysis and energy dispersive spectroscopy revealed heterogeneity of mineral elements across body cuticle and a concentration of ACP in the epicuticle, especially associated with the hemispherical structures. Further imaging and analysis showed the bulk of the ACP to be present in nano-sized granules. It is hypothesised that the specific distribution of ACP may enhance cuticular hardness or durability without reducing flexibility. q 2004 Elsevier Ltd. All rights reserved. Keywords: Insect; Cuticle; Integument; Hardening; Analytical electron microscopy; Electron microprobe 1. Introduction was distributed heterogeneously. Further investigation of the distribution of the mineral phase at the micron and Strengthening of biological structures through cuticular nanometre level is needed to discover where deposition is calcification is well developed in decapod crustaceans but it occurring and how this might affect exoskeletal organis- rarely occurs in insects, where it is poorly understood ation. -

A Perspective on Underlying Crystal Growth Mechanisms in Biomineralization: Solution Mediated Growth Versus Nanosphere Particle Accretion
CrystEngComm A perspective on underlying crystal growth mechanisms in biomineralization: solution mediated growth versus nanosphere particle accretion Journal: CrystEngComm Manuscript ID: CE-HIG-07-2014-001474.R1 Article Type: Highlight Date Submitted by the Author: 01-Dec-2014 Complete List of Authors: Gal, Assaf; Weizmann Institute of Science, Structural Biology Weiner, Steve; Weizmann Institute of Science, Structural Biology Addadi, Lia; Weizmann Institute of Science, Structural Biology Page 1 of 23 CrystEngComm A perspective on underlying crystal growth mechanisms in biomineralization: solution mediated growth versus nanosphere particle accretion Assaf Gal, Steve Weiner, and Lia Addadi Department of Structural Biology, Weizmann Institute of Science, Rehovot, Israel 76100 Abstract Many organisms form crystals from transient amorphous precursor phases. In the cases where the precursor phases were imaged, they consist of nanosphere particles. Interestingly, some mature biogenic crystals also have nanosphere particle morphology, but some are characterized by crystallographic faces that are smooth at the nanometer level. There are also biogenic crystals that have both crystallographic faces and nanosphere particle morphology. This highlight presents a working hypothesis, stating that some biomineralization processes involve growth by nanosphere particle accretion, where amorphous nanoparticles are incorporated as such into growing crystals and preserve their morphology upon crystallization. This process produces biogenic crystals with a nanosphere particle morphology. Other biomineralization processes proceed by ion-by-ion growth, and some cases of biological crystal growth involve both processes. We also identify several biomineralization processes which do not seem to fit this working hypothesis. It is our hope that this highlight will inspire studies that will shed more light on the underlying crystallization mechanisms in biology. -

An Overview of Biomineralization Processes and the Problem of The
11 An Overview of Biomineralization Processes and the Problem of the Vital Effect Steve Weiner Department of Structural Biology Weizmann Institute of Science 76100 Rehovot Israel Patricia M. Dove Department of GeoSciences Virginia Tech Blacksburg, Virginia 24061 U.S.A. “Biomineralization links soft organic tissues, which are compositionally akin to the atmosphere and oceans, with the hard materials of the solid Earth. It provides organisms with skeletons and shells while they are alive, and when they die these are deposited as sediment in environments from river plains to the deep ocean floor. It is also these hard, resistant products of life which are mainly responsible for the Earth’s fossil record. Consequently, biomineralization involves biologists, chemists, and geologists in interdisciplinary studies at one of the interfaces between Earth and life.” (Leadbeater and Riding 1986) INTRODUCTION Biomineralization refers to the processes by which organisms form minerals. The control exerted by many organisms over mineral formation distinguishes these processes from abiotic mineralization. The latter was the primary focus of earth scientists over the last century, but the emergence of biogeochemistry and the urgency of understanding the past and future evolution of the Earth are moving biological mineralization to the forefront of various fields of science, including the earth sciences. The growth in biogeochemistry has led to a number of new exciting research areas where the distinctions between the biological, chemical, and earth sciences disciplines melt away. Of the wonderful topics that are receiving renewed attention, the study of biomineral formation is perhaps the most fascinating. Truly at the interface of earth and life, biomineralization is a discipline that is certain to see major advancements as a new generation of scientists brings cross-disciplinary training and new experimental and computational methods to the most daunting problems. -

Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs
Effects of Ocean Acidification and Sea-Level Rise on Coral Reefs Coral reefs are vital to the long-term to produce CaCO3, carbon dioxide (CO2), As CO2 increases in the atmosphere, viability of coastal societies, providing and water (H2O). Over time as these more is absorbed by the surface of the economic, recreational, and aesthetic organisms grow and die, their skeletons ocean, where it combines with seawater value from which coastal communities break down and become calcium carbon- to make a weak acid called carbonic thrive. Some of the services that coral ate sediments. These sediments fill in acid (H2CO3). This process, called reefs provide include protection from the framework of the reef and eventually ocean acidification, causes a decrease storm waves, nurseries and habitats for become cemented together, construct- in seawater pH (or increase in acidity) commercially important fish species, ing the foundation for continued upward that can result in a decrease in biogenic and production of sand for beaches. growth of the reef structure. The infilling calcification rates, dissolution of carbon- Coral reefs develop over thousands of the reef framework with sediments is ate sediments, and loss of reef structure. of years as tropical marine organ- what allows vertical accretion over time One of the primary concerns associated isms build skeletons of calcium carbonate and enables reef growth to keep up with with ocean acidification is whether coral (CaCO3) minerals to form a three-dimen- sea-level rise. Calcification is a revers- reefs will be able to continue to grow at sional structure (fig. 1). This process, ible process.