Gelatinous Zooplankton‐Mediated Carbon Flows In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Reforestation in Carbon Sequestration
New Forests (2019) 50:115–137 https://doi.org/10.1007/s11056-018-9655-3 The role of reforestation in carbon sequestration L. E. Nave1,2 · B. F. Walters3 · K. L. Hofmeister4 · C. H. Perry3 · U. Mishra5 · G. M. Domke3 · C. W. Swanston6 Received: 12 October 2017 / Accepted: 24 June 2018 / Published online: 9 July 2018 © Springer Nature B.V. 2018 Abstract In the United States (U.S.), the maintenance of forest cover is a legal mandate for federally managed forest lands. More broadly, reforestation following harvesting, recent or historic disturbances can enhance numerous carbon (C)-based ecosystem services and functions. These include production of woody biomass for forest products, and mitigation of atmos- pheric CO2 pollution and climate change by sequestering C into ecosystem pools where it can be stored for long timescales. Nonetheless, a range of assessments and analyses indicate that reforestation in the U.S. lags behind its potential, with the continuation of ecosystem services and functions at risk if reforestation is not increased. In this context, there is need for multiple independent analyses that quantify the role of reforestation in C sequestration, from ecosystems up to regional and national levels. Here, we describe the methods and report the fndings of a large-scale data synthesis aimed at four objectives: (1) estimate C storage in major ecosystem pools in forest and other land cover types; (2) quan- tify sources of variation in ecosystem C pools; (3) compare the impacts of reforestation and aforestation on C pools; (4) assess whether these results hold or diverge across ecore- gions. -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
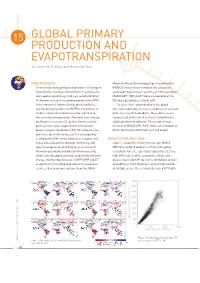
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Require
Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jorge Andres Villa Betancur Graduate Program in Environmental Science The Ohio State University 2014 Dissertation Committee: William J. Mitsch, Co-advisor Gil Bohrer, Co-advisor James Bauer Jay Martin Copyrighted by Jorge Andres Villa Betancur 2014 Abstract Emission and uptake of greenhouse gases and the production and transport of dissolved organic matter in different wetland plant communities are key wetland functions determining two important ecosystem services, climate regulation and nutrient cycling. The objective of this dissertation was to study the variation of methane emissions, carbon sequestration and exports of dissolved organic carbon in wetland plant communities of a subtropical climate in south Florida. The plant communities selected for the study of methane emissions and carbon sequestration were located in a natural wetland landscape and corresponded to a gradient of inundation duration. Going from the wettest to the driest conditions, the communities were designated as: deep slough, bald cypress, wet prairie, pond cypress and hydric pine flatwood. In the first methane emissions study, non-steady-state rigid chambers were deployed at each community sequentially at three different times of the day during a 24- month period. Methane fluxes from the different communities did not show a discernible daily pattern, in contrast to a marked increase in seasonal emissions during inundation. All communities acted at times as temporary sinks for methane, but overall were net -2 -1 sources. Median and mean + standard error fluxes in g CH4-C.m .d were higher in the deep slough (11 and 56.2 + 22.1), followed by the wet prairie (9.01 and 53.3 + 26.6), bald cypress (3.31 and 5.54 + 2.51) and pond cypress (1.49, 4.55 + 3.35) communities. -

Net Ecosystem Production: a Comprehensive Measure of Net Carbon Accumulation by Ecosystems
Ecological Applications, 12(4), 2002, pp. 937±947 q 2002 by the Ecological Society of America NET ECOSYSTEM PRODUCTION: A COMPREHENSIVE MEASURE OF NET CARBON ACCUMULATION BY ECOSYSTEMS J. T. RANDERSON,1,6 F. S . C HAPIN, III,2 J. W. HARDEN,3 J. C. NEFF,4 AND M. E. HARMON5 1Divisions of Engineering and Applied Science and Geological and Planetary Sciences, California Institute of Technology, Mail Stop 100-23, Pasadena, California 91125 USA 2Institute for Arctic Biology, 311 Irvine 1 Building, University of Alaska, Fairbanks, Fairbanks, Alaska, 99775 USA 3U.S. Geological Survey, Mail Stop 962, 345 Middle®eld Road, Menlo Park, California 94025 USA 4U.S. Geological Survey, Mail Stop 980, Denver Federal Center, Denver, Colorado 80225 USA 5Department of Forest Science, Oregon State University, Corvallis, Oregon 97331-57521 USA Abstract. The conceptual framework used by ecologists and biogeochemists must allow for accurate and clearly de®ned comparisons of carbon ¯uxes made with disparate tech- niques across a spectrum of temporal and spatial scales. Consistent with usage over the past four decades, we de®ne ``net ecosystem production'' (NEP) as the net carbon accu- mulation by ecosystems. Past use of this term has been ambiguous, because it has been used conceptually as a measure of carbon accumulation by ecosystems, but it has often been calculated considering only the balance between gross primary production (GPP) and ecosystem respiration. This calculation ignores other carbon ¯uxes from ecosystems (e.g., leaching of dissolved carbon and losses associated with disturbance). To avoid conceptual ambiguities, we argue that NEP be de®ned, as in the past, as the net carbon accumulation by ecosystems and that it explicitly incorporate all the carbon ¯uxes from an ecosystem, including autotrophic respiration, heterotrophic respiration, losses associated with distur- bance, dissolved and particulate carbon losses, volatile organic compound emissions, and lateral transfers among ecosystems. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

From Nano-Gels to Marine Snow: a Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean
gels Review From Nano-Gels to Marine Snow: A Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean Antonietta Quigg 1,* , Peter H. Santschi 2 , Adrian Burd 3, Wei-Chun Chin 4 , Manoj Kamalanathan 1, Chen Xu 2 and Kai Ziervogel 5 1 Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] 2 Department of Marine and Coastal Environmental Science, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] (P.H.S.); [email protected] (C.X.) 3 Department of Marine Science, University of Georgia, Athens, GA 30602, USA; [email protected] 4 Department of Bioengineering, University of California, Merced, CA 95343, USA; [email protected] 5 Institute for the Study of Earth, Oceans and Space, University of New Hampshire, Durham, NH 03824, USA; [email protected] * Correspondence: [email protected] Abstract: Marine gels (nano-, micro-, macro-) and marine snow play important roles in regulating global and basin-scale ocean biogeochemical cycling. Exopolymeric substances (EPS) including transparent exopolymer particles (TEP) that form from nano-gel precursors are abundant materials in the ocean, accounting for an estimated 700 Gt of carbon in seawater. This supports local microbial communities that play a critical role in the cycling of carbon and other macro- and micro-elements in Citation: Quigg, A.; Santschi, P.H.; the ocean. Recent studies have furthered our understanding of the formation and properties of these Burd, A.; Chin, W.-C.; Kamalanathan, materials, but the relationship between the microbial polymers released into the ocean and marine M.; Xu, C.; Ziervogel, K. -

Trends in Soil Solution Dissolved Organic Carbon (DOC) Concentrations Across European Forests
Biogeosciences, 13, 5567–5585, 2016 www.biogeosciences.net/13/5567/2016/ doi:10.5194/bg-13-5567-2016 © Author(s) 2016. CC Attribution 3.0 License. Trends in soil solution dissolved organic carbon (DOC) concentrations across European forests Marta Camino-Serrano1, Elisabeth Graf Pannatier2, Sara Vicca1, Sebastiaan Luyssaert3,a, Mathieu Jonard4, Philippe Ciais3, Bertrand Guenet3, Bert Gielen1, Josep Peñuelas5,6, Jordi Sardans5,6, Peter Waldner2, Sophia Etzold2, Guia Cecchini7, Nicholas Clarke8, Zoran Galic´9, Laure Gandois10, Karin Hansen11, Jim Johnson12, Uwe Klinck13, Zora Lachmanová14, Antti-Jussi Lindroos15, Henning Meesenburg13, Tiina M. Nieminen15, Tanja G. M. Sanders16, Kasia Sawicka17, Walter Seidling16, Anne Thimonier2, Elena Vanguelova18, Arne Verstraeten19, Lars Vesterdal20, and Ivan A. Janssens1 1Research Group of Plant and Vegetation Ecology, Department of Biology, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk, Belgium 2WSL, Swiss Federal Institute for Forest, Snow and Landscape Research, Zürcherstrasse 111, 8903, Birmensdorf, Switzerland 3Laboratoire des Sciences du Climat et de l’Environnement, LSCE/IPSL, CEA-CNRS-UVSQ, Université Paris-Saclay, 91191 Gif-sur-Yvette, France 4UCL-ELI, Université catholique de Louvain, Earth and Life Institute, Croix du Sud 2, 1348 Louvain-la-Neuve, Belgium 5CREAF, Cerdanyola del Vallès, 08193, Catalonia, Spain 6CSIC, Global Ecology Unit CREAF-CSIC-UAB, Cerdanyola del Vallès, 08193, Catalonia, Spain 7Department of Earth Sciences, University of Florence, Via La Pira 4, 50121 Florence, -

Trophic Ecology of Gelatinous Zooplankton in Oceanic Food Webs of the Eastern Tropical Atlantic Assessed by Stable Isotope Analysis
Limnol. Oceanogr. 9999, 2020, 1–17 © 2020 The Authors. Limnology and Oceanography published by Wiley Periodicals LLC on behalf of Association for the Sciences of Limnology and Oceanography. doi: 10.1002/lno.11605 Tackling the jelly web: Trophic ecology of gelatinous zooplankton in oceanic food webs of the eastern tropical Atlantic assessed by stable isotope analysis Xupeng Chi ,1,2* Jan Dierking,2 Henk-Jan Hoving,2 Florian Lüskow,3,4 Anneke Denda,5 Bernd Christiansen,5 Ulrich Sommer,2 Thomas Hansen,2 Jamileh Javidpour2,6 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China 2Marine Ecology, GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany 3Department of Earth, Ocean and Atmospheric Sciences, University of British Columbia, Vancouver, British Columbia, Canada 4Institute for the Oceans and Fisheries, University of British Columbia, Vancouver, British Columbia, Canada 5Institute of Marine Ecosystem and Fishery Science (IMF), Universität Hamburg, Hamburg, Germany 6Department of Biology, University of Southern Denmark, Odense M, Denmark Abstract Gelatinous zooplankton can be present in high biomass and taxonomic diversity in planktonic oceanic food webs, yet the trophic structuring and importance of this “jelly web” remain incompletely understood. To address this knowledge gap, we provide a holistic trophic characterization of a jelly web in the eastern tropical Atlantic, based on δ13C and δ15N stable isotope analysis of a unique gelatinous zooplankton sample set. The jelly web covered most of the isotopic niche space of the entire planktonic oceanic food web, spanning > 3 tro- phic levels, ranging from herbivores (e.g., pyrosomes) to higher predators (e.g., ctenophores), highlighting the diverse functional roles and broad possible food web relevance of gelatinous zooplankton. -

Soil Carbon Sequestration and Greenhouse Gas Mitigation: a Role
Soil Carbon Sequestration and Greenhouse Gas Mitigation: A Role for American Agriculture Dr. Charles W. Rice and Debbie Reed, MSc. Professor of Agronomy Kansas State University Department of Agronomy Manhattan, KS Agriculture and Climate Change Specialist DRD Associates Arlington, VA March 2007 1 TABLE OF CONTENTS Executive Summary (pg. 5) Global Climate Change (7) Greenhouse Gases and American Agriculture (8) The Role of Agriculture in Combating Rising Greenhouse Gas Emissions and Climate Change (8) Agricultural Practices that Combat Climate Change (10) Decreasing emissions (10) Enhancing sinks (10) Displacing emissions (11) How American Agriculture Can Reduce U.S. GHG Emissions and Combat Climate Change (multi-page spread: 12-14) Building Carbon Sinks: Soil Carbon Sequestration (12) Cropland (12) Agronomy (12) Nutrient Management (12) Tillage/Residue Management (13) Land cover/land use change (13) Grazingland management and pasture improvement (13) Grazing intensity (13) Increased productivity (including fertilization) (13) Nutrient management (13) Fire management (13) Restoration of degraded lands (13) Decreasing GHG Emissions: Manure Management (14) Displacing GHG Emissions: Biofuels (14) Table 1: Estimates of potential carbon sequestration of agricultural practices (14) Economic Benefits of Conservation Tillage and No-Till Systems (15) Table 2: Change in yield, net dollar returns, emissions, and soil carbon when converting from conventional tillage to no tillage corn production in Northeast Kansas (15) Co-Benefits of Soil Carbon Sequestration: “Charismatic Carbon” (16) Table 3: Plant nutrients supplied by soil organic matter (SOM) (16) 2 New Technologies to Further Enhance Soil Carbon Storage (16) Biochar (16) Modification of the Plant-Soil System (17) Meeting the Climate Challenge: The Role of Carbon Markets (17) Mandatory v. -

Ocean Storage
277 6 Ocean storage Coordinating Lead Authors Ken Caldeira (United States), Makoto Akai (Japan) Lead Authors Peter Brewer (United States), Baixin Chen (China), Peter Haugan (Norway), Toru Iwama (Japan), Paul Johnston (United Kingdom), Haroon Kheshgi (United States), Qingquan Li (China), Takashi Ohsumi (Japan), Hans Pörtner (Germany), Chris Sabine (United States), Yoshihisa Shirayama (Japan), Jolyon Thomson (United Kingdom) Contributing Authors Jim Barry (United States), Lara Hansen (United States) Review Editors Brad De Young (Canada), Fortunat Joos (Switzerland) 278 IPCC Special Report on Carbon dioxide Capture and Storage Contents EXECUTIVE SUMMARY 279 6.7 Environmental impacts, risks, and risk management 298 6.1 Introduction and background 279 6.7.1 Introduction to biological impacts and risk 298 6.1.1 Intentional storage of CO2 in the ocean 279 6.7.2 Physiological effects of CO2 301 6.1.2 Relevant background in physical and chemical 6.7.3 From physiological mechanisms to ecosystems 305 oceanography 281 6.7.4 Biological consequences for water column release scenarios 306 6.2 Approaches to release CO2 into the ocean 282 6.7.5 Biological consequences associated with CO2 6.2.1 Approaches to releasing CO2 that has been captured, lakes 307 compressed, and transported into the ocean 282 6.7.6 Contaminants in CO2 streams 307 6.2.2 CO2 storage by dissolution of carbonate minerals 290 6.7.7 Risk management 307 6.2.3 Other ocean storage approaches 291 6.7.8 Social aspects; public and stakeholder perception 307 6.3 Capacity and fractions retained