Microbial Loop Carbon Cycling in Ocean Environments Studied Using a Simple Steady-State Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Response of Marine Food Webs to Climate-Induced Changes in Temperature and Inflow of Allochthonous Organic Matter
Response of marine food webs to climate-induced changes in temperature and inflow of allochthonous organic matter Rickard Degerman Department of Ecology and Environmental Science 901 87 Umeå Umeå 2015 1 Copyright©Rickard Degerman ISBN: 978-91-7601-266-6 Front cover illustration by Mats Minnhagen Printed by: KBC Service Center, Umeå University Umeå, Sweden 2015 2 Tillägnad Maria, Emma och Isak 3 Table of Contents Abstract 5 List of papers 6 Introduction 7 Aquatic food webs – different pathways Food web efficiency – a measure of ecosystem function Top predators cause cascade effects on lower trophic levels The Baltic Sea – a semi-enclosed sea exposed to multiple stressors Varying food web structures Climate-induced changes in the marine ecosystem Food web responses to increased temperature Responses to inputs of allochthonous organic matter Objectives 14 Material and Methods 14 Paper I Paper II and III Paper IV Results and Discussion 18 Effect of temperature and nutrient availability on heterotrophic bacteria Influence of food web length and labile DOC on pelagic productivity and FWE Consequences of changes in inputs of ADOM and temperature for pelagic productivity and FWE Control of pelagic productivity, FWE and ecosystem trophic balance by colored DOC Conclusion and future perspectives 21 Author contributions 23 Acknowledgements 23 Thanks 24 References 25 4 Abstract Global records of temperature show a warming trend both in the atmosphere and in the oceans. Current climate change scenarios indicate that global temperature will continue to increase in the future. The effects will however be very different in different geographic regions. In northern Europe precipitation is projected to increase along with temperature. -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
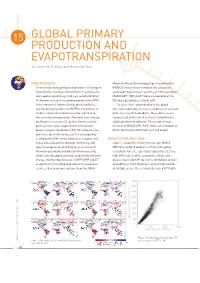
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Require
Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jorge Andres Villa Betancur Graduate Program in Environmental Science The Ohio State University 2014 Dissertation Committee: William J. Mitsch, Co-advisor Gil Bohrer, Co-advisor James Bauer Jay Martin Copyrighted by Jorge Andres Villa Betancur 2014 Abstract Emission and uptake of greenhouse gases and the production and transport of dissolved organic matter in different wetland plant communities are key wetland functions determining two important ecosystem services, climate regulation and nutrient cycling. The objective of this dissertation was to study the variation of methane emissions, carbon sequestration and exports of dissolved organic carbon in wetland plant communities of a subtropical climate in south Florida. The plant communities selected for the study of methane emissions and carbon sequestration were located in a natural wetland landscape and corresponded to a gradient of inundation duration. Going from the wettest to the driest conditions, the communities were designated as: deep slough, bald cypress, wet prairie, pond cypress and hydric pine flatwood. In the first methane emissions study, non-steady-state rigid chambers were deployed at each community sequentially at three different times of the day during a 24- month period. Methane fluxes from the different communities did not show a discernible daily pattern, in contrast to a marked increase in seasonal emissions during inundation. All communities acted at times as temporary sinks for methane, but overall were net -2 -1 sources. Median and mean + standard error fluxes in g CH4-C.m .d were higher in the deep slough (11 and 56.2 + 22.1), followed by the wet prairie (9.01 and 53.3 + 26.6), bald cypress (3.31 and 5.54 + 2.51) and pond cypress (1.49, 4.55 + 3.35) communities. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Microbial Loop' in Stratified Systems
MARINE ECOLOGY PROGRESS SERIES Vol. 59: 1-17, 1990 Published January 11 Mar. Ecol. Prog. Ser. 1 A steady-state analysis of the 'microbial loop' in stratified systems Arnold H. Taylor, Ian Joint Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth PLl 3DH, United Kingdom ABSTRACT. Steady state solutions are presented for a simple model of the surface mixed layer, which contains the components of the 'microbial loop', namely phytoplankton, picophytoplankton, bacterio- plankton, microzooplankton, dissolved organic carbon, detritus, nitrate and ammonia. This system is assumed to be in equilibrium with the larger grazers present at any time, which are represented as an external mortality function. The model also allows for dissolved organic nitrogen consumption by bacteria, and self-grazing and mixotrophy of the microzooplankton. The model steady states are always stable. The solution shows a number of general properties; for example, biomass of each individual component depends only on total nitrogen concentration below the mixed layer, not whether the nitrogen is in the form of nitrate or ammonia. Standing stocks and production rates from the model are compared with summer observations from the Celtic Sea and Porcupine Sea Bight. The agreement is good and suggests that the system is often not far from equilibrium. A sensitivity analysis of the model is included. The effect of varying the mixing across the pycnocline is investigated; more intense mixing results in the large phytoplankton population increasing at the expense of picophytoplankton, micro- zooplankton and DOC. The change from phytoplankton to picophytoplankton dominance at low mixing occurs even though the same physiological parameters are used for both size fractions. -

From Nano-Gels to Marine Snow: a Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean
gels Review From Nano-Gels to Marine Snow: A Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean Antonietta Quigg 1,* , Peter H. Santschi 2 , Adrian Burd 3, Wei-Chun Chin 4 , Manoj Kamalanathan 1, Chen Xu 2 and Kai Ziervogel 5 1 Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] 2 Department of Marine and Coastal Environmental Science, Texas A&M University at Galveston, Galveston, TX 77553, USA; [email protected] (P.H.S.); [email protected] (C.X.) 3 Department of Marine Science, University of Georgia, Athens, GA 30602, USA; [email protected] 4 Department of Bioengineering, University of California, Merced, CA 95343, USA; [email protected] 5 Institute for the Study of Earth, Oceans and Space, University of New Hampshire, Durham, NH 03824, USA; [email protected] * Correspondence: [email protected] Abstract: Marine gels (nano-, micro-, macro-) and marine snow play important roles in regulating global and basin-scale ocean biogeochemical cycling. Exopolymeric substances (EPS) including transparent exopolymer particles (TEP) that form from nano-gel precursors are abundant materials in the ocean, accounting for an estimated 700 Gt of carbon in seawater. This supports local microbial communities that play a critical role in the cycling of carbon and other macro- and micro-elements in Citation: Quigg, A.; Santschi, P.H.; the ocean. Recent studies have furthered our understanding of the formation and properties of these Burd, A.; Chin, W.-C.; Kamalanathan, materials, but the relationship between the microbial polymers released into the ocean and marine M.; Xu, C.; Ziervogel, K. -

Trends in Soil Solution Dissolved Organic Carbon (DOC) Concentrations Across European Forests
Biogeosciences, 13, 5567–5585, 2016 www.biogeosciences.net/13/5567/2016/ doi:10.5194/bg-13-5567-2016 © Author(s) 2016. CC Attribution 3.0 License. Trends in soil solution dissolved organic carbon (DOC) concentrations across European forests Marta Camino-Serrano1, Elisabeth Graf Pannatier2, Sara Vicca1, Sebastiaan Luyssaert3,a, Mathieu Jonard4, Philippe Ciais3, Bertrand Guenet3, Bert Gielen1, Josep Peñuelas5,6, Jordi Sardans5,6, Peter Waldner2, Sophia Etzold2, Guia Cecchini7, Nicholas Clarke8, Zoran Galic´9, Laure Gandois10, Karin Hansen11, Jim Johnson12, Uwe Klinck13, Zora Lachmanová14, Antti-Jussi Lindroos15, Henning Meesenburg13, Tiina M. Nieminen15, Tanja G. M. Sanders16, Kasia Sawicka17, Walter Seidling16, Anne Thimonier2, Elena Vanguelova18, Arne Verstraeten19, Lars Vesterdal20, and Ivan A. Janssens1 1Research Group of Plant and Vegetation Ecology, Department of Biology, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk, Belgium 2WSL, Swiss Federal Institute for Forest, Snow and Landscape Research, Zürcherstrasse 111, 8903, Birmensdorf, Switzerland 3Laboratoire des Sciences du Climat et de l’Environnement, LSCE/IPSL, CEA-CNRS-UVSQ, Université Paris-Saclay, 91191 Gif-sur-Yvette, France 4UCL-ELI, Université catholique de Louvain, Earth and Life Institute, Croix du Sud 2, 1348 Louvain-la-Neuve, Belgium 5CREAF, Cerdanyola del Vallès, 08193, Catalonia, Spain 6CSIC, Global Ecology Unit CREAF-CSIC-UAB, Cerdanyola del Vallès, 08193, Catalonia, Spain 7Department of Earth Sciences, University of Florence, Via La Pira 4, 50121 Florence, -

Deciphering Ocean Carbon in a Changing World PERSPECTIVE Mary Ann Morana,1, Elizabeth B
PERSPECTIVE Deciphering ocean carbon in a changing world PERSPECTIVE Mary Ann Morana,1, Elizabeth B. Kujawinskib,1, Aron Stubbinsc,1, Rob Fatlandd,1, Lihini I. Aluwiharee, Alison Buchanf, Byron C. Crumpg, Pieter C. Dorresteinh,i,j, Sonya T. Dyhrmank,l, Nancy J. Hessm, Bill Howen, Krista Longneckerb, Patricia M. Medeirosa, Jutta Niggemanno, Ingrid Obernostererp, Daniel J. Repetab, and Jacob R. Waldbauerq Edited by David M. Karl, University of Hawaii, Honolulu, HI, and approved February 11, 2016 (received for review October 21, 2015) Dissolved organic matter (DOM) in the oceans is one of the largest pools of reduced carbon on Earth, comparable in size to the atmospheric CO2 reservoir. A vast number of compounds are present in DOM, and they play important roles in all major element cycles, contribute to the storage of atmospheric CO2 in the ocean, support marine ecosystems, and facilitate interactions between organisms. At the heart of the DOM cycle lie molecular-level relationships between the individual compounds in DOM and the members of the ocean microbiome that produce and consume them. In the past, these connections have eluded clear definition because of the sheer numerical complexity of both DOM molecules and microorganisms. Emerging tools in analytical chemistry, microbiology, and informatics are breaking down the barriers to a fuller appreciation of these connections. Here we highlight questions being addressed using recent meth- odological and technological developments in those fields and consider how these advances are trans- forming our understanding of some of the most important reactions of the marine carbon cycle. dissolved organic matter | marine microbes | cyberinfrastructure The global cycling of carbon supports life on Earth and and fundamental interactions have been necessarily affects the state of the biosphere within which humans oversimplified to yield a scientifically tractable reside. -

Dissolved Organic Carbon (DOC) (For Private Water and Health Regulated Public Water Supplies)
Dissolved Organic Carbon (DOC) (For Private Water and Health Regulated Public Water Supplies) What Is Dissolved Organic Carbon? Dissolved organic carbon (DOC) is a general description of the organic material dissolved in water. Organic carbon occurs as the result of decomposition of plant or animal material. Organic carbon present in soil or water bodies may then dissolve when contacted by water. This dissolved organic carbon moves with both surface water and ground water. Acknowledgement: How Does Dissolved Organic Carbon Get Into Water? Organic material (including carbon) results from decomposition of plants or animals. This Fact Sheet is one of a Once this decomposed organic material contacts water it may partially dissolve. series developed by an Interagency Committee with representatives from How Does Dissolved Organic Carbon Affect My Health? Saskatchewan Ministry of DOC does not pose health risk itself but may become potentially harmful when in Health, Regional Health combination with other aspects of your water. When water with high DOC is Authorities, Saskatchewan chlorinated, harmful byproducts called trihalomethanes may be produced (see Watershed Authority, SaskH2O factsheet on trihalomethanes). Trihalomethanes may have long-term Saskatchewan Ministry of effects on health and they should be considered when chlorinating drinking water Environment, Saskatchewan Ministry of Agriculture, high in DOC. According to Health Canada, the benefits of chlorinating drinking Agriculture and Agri-Food water are much greater than the health risks associated with chlorination by- Canada – PFRA and Health products such as trihalomethanes Canada. DOC can interfere with the effectiveness of disinfection processes such as Responsibility for chlorination, ultraviolet and ozone sterilization. DOC can also promote the growth of interpretation of the content of microorganisms by providing a food source. -

Developments in Aquatic Microbiology
INTERNATL MICROBIOL (2000) 3: 203–211 203 © Springer-Verlag Ibérica 2000 REVIEW ARTICLE Samuel P. Meyers Developments in aquatic Department of Oceanography and Coastal Sciences, Louisiana State University, microbiology Baton Rouge, LA, USA Received 30 August 2000 Accepted 29 September 2000 Summary Major discoveries in marine microbiology over the past 4-5 decades have resulted in the recognition of bacteria as a major biomass component of marine food webs. Such discoveries include chemosynthetic activities in deep-ocean ecosystems, survival processes in oligotrophic waters, and the role of microorganisms in food webs coupled with symbiotic relationships and energy flow. Many discoveries can be attributed to innovative methodologies, including radioisotopes, immunofluores- cent-epifluorescent analysis, and flow cytometry. The latter has shown the key role of marine viruses in marine system energetics. Studies of the components of the “microbial loop” have shown the significance of various phagotrophic processes involved in grazing by microinvertebrates. Microbial activities and dissolved organic carbon are closely coupled with the dynamics of fluctuating water masses. New biotechnological approaches and the use of molecular biology techniques still provide new and relevant information on the role of microorganisms in oceanic and estuarine environments. International interdisciplinary studies have explored ecological aspects of marine microorganisms and their significance in biocomplexity. Studies on the Correspondence to: origins of both life and ecosystems now focus on microbiological processes in the Louisiana State University Station. marine environment. This paper describes earlier and recent discoveries in marine Post Office Box 19090-A. Baton Rouge, LA 70893. USA (aquatic) microbiology and the trends for future work, emphasizing improvements Tel.: +1-225-3885180 in methodology as major catalysts for the progress of this broadly-based field.