Sinking Jelly-Carbon Unveils Potential Environmental Variability Along a Continental Margin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Total Organic Carbon (TOC) Guidance Manual
September 2002 RG-379 (Revised) Total Organic Carbon (TOC) Guidance Manual Water Supply Division printed on recycled paper TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Total Organic Carbon (TOC) Guidance Manual Prepared by Water Supply Division RG-379 (Revised) September 2002 Robert J. Huston, Chairman R. B. “Ralph” Marquez, Commissioner Kathleen Hartnett White, Commissioner Jeffrey A. Saitas, Executive Director Authorization to use or reproduce any original material contained in this publication—that is, not obtained from other sources—is freely granted. The commission would appreciate acknowledgment. Copies of this publication are available for public use through the Texas State Library, other state depository libraries, and the TCEQ Library, in compli- ance with state depository law. For more information on TCEQ publications call 512/239-0028 or visit our Web site at: www.tceq.state.tx.us/publications Published and distributed by the Texas Commission on Environmental Quality PO Box 13087 Austin TX 78711-3087 The Texas Commission on Environmental Quality was formerly called the Texas Natural Resource Conservation Commission. The TCEQ is an equal opportunity/affirmative action employer. The agency does not allow discrimination on the basis of race, color, religion, national origin, sex, disability, age, sexual orientation or veteran status. In compliance with the Americans with Disabilities Act, this document may be requested in alternate formats by contacting the TCEQ at 512/239-0028, Fax 239-4488, or 1-800-RELAY-TX (TDD), or by writing -

Estimation of Atmospheric Total Organic Carbon (TOC) – Paving The
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1055 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 12 October 2018 c Author(s) 2018. CC BY 4.0 License. Estimation of atmospheric total organic carbon (TOC) – paving the path towards carbon budget closure Mingxi Yang1*, Zoë L. Fleming2& 5 1 Plymouth Marine Laboratory, Plymouth, United Kingdom 2 National Centre for Atmospheric Science (NCAS), Department of Chemistry, University of Leicester, UK, United Kingdom * Correspondence to M. Yang ([email protected]) & Now at Center for Climate and Resilience Research (CR2), Departamento de Geofísica, Universidad de Chile, Santiago, Chile 10 Abstract. The atmosphere contains a rich variety of reactive organic compounds, including gaseous volatile organic carbon (VOCs), carbonaceous aerosols, and other organic compounds at varying volatility. Here we present measurements of atmospheric non-methane total organic carbon plus carbon monoxide (TOC+CO) during August-September 2016 from a coastal 15 city in the southwest United Kingdom. TOC+CO was substantially elevated during the day on weekdays (occasionally over 2 ppm C) as a result of local anthropogenic activity. On weekends and holidays, with a mean (standard error) of 102 (8) ppb C, TOC+CO was lower and showed much less diurnal variability. Excluding weekday daytime, TOC+CO was significantly lower when winds were coming off the Atlantic Ocean than when winds were coming off land. By subtracting the estimated CO from TOC+CO, we constrain the mean (uncertainty) TOC in marine air to be around 19 (±≥8) ppb C during this period. A proton- 20 transfer-reaction mass spectrometer (PTR-MS) was deployed at the same time, detecting a large range of organic compounds (oxygenated VOCs, biogenic VOCs, aromatics, dimethyl sulfide). -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

E.’S Responses ~(Continued)
: I’able K-3. ,Scnping stat~nts and EIS sectimns or .~E.’s responses ~(continued) COnunent scoping nuinber :Stateilmnt topic EIS section or DOE comnt h at this tti want to reiter&e that the Enviro~ntal l~act Statement should not represent. a legalistic charade ..but a sin. cere commitment to seek and evaluate pertinent in for~t ion. Obviously, any Envirocnnental. Assemnt- which led to the Find- ing of No Significant .l~act needs to be reviewed, evaluated and expanded upon, with full regard to the in~t of a broad range of interests, incl”di”g state agenciea,. the: academic comunit y, public interest groups, and private c iti-zens. We muld like to offer some co-”ts: on the in format io” euppl=ied by 00E relative to the probable contents. of the EIS. In the category of production alternatives: : It wuld seem L Need .Section 1.1 ., important tO. re-evaluate the ,need for i~reased protict ion,~nd %e Commnt D1 make every attempt to ::scale dom those need8. It is ine8ca~ able that the quest ion. af the.,need .to prodca plutoniun ia part of the greater ongoing natic,”al.?security debate. If it is indeed. essential that plutoniun: probct ion be stepped up, the Alternatives Saction 2.1 viable? alternatives should be thoroughly explored in the EIS. In the category of socioeconamics: A broad consideration of %cioeconomic .%ction 5.2.1 the state needs to be incorporated, beyond the immdiate jobs effects :-at SiU during construction and as .an ongoing operation. South Carolina has trenmndous potential for no-nuclear economic. -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
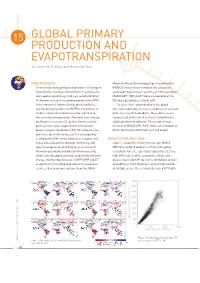
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Require
Carbon Dynamics of Subtropical Wetland Communities in South Florida DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jorge Andres Villa Betancur Graduate Program in Environmental Science The Ohio State University 2014 Dissertation Committee: William J. Mitsch, Co-advisor Gil Bohrer, Co-advisor James Bauer Jay Martin Copyrighted by Jorge Andres Villa Betancur 2014 Abstract Emission and uptake of greenhouse gases and the production and transport of dissolved organic matter in different wetland plant communities are key wetland functions determining two important ecosystem services, climate regulation and nutrient cycling. The objective of this dissertation was to study the variation of methane emissions, carbon sequestration and exports of dissolved organic carbon in wetland plant communities of a subtropical climate in south Florida. The plant communities selected for the study of methane emissions and carbon sequestration were located in a natural wetland landscape and corresponded to a gradient of inundation duration. Going from the wettest to the driest conditions, the communities were designated as: deep slough, bald cypress, wet prairie, pond cypress and hydric pine flatwood. In the first methane emissions study, non-steady-state rigid chambers were deployed at each community sequentially at three different times of the day during a 24- month period. Methane fluxes from the different communities did not show a discernible daily pattern, in contrast to a marked increase in seasonal emissions during inundation. All communities acted at times as temporary sinks for methane, but overall were net -2 -1 sources. Median and mean + standard error fluxes in g CH4-C.m .d were higher in the deep slough (11 and 56.2 + 22.1), followed by the wet prairie (9.01 and 53.3 + 26.6), bald cypress (3.31 and 5.54 + 2.51) and pond cypress (1.49, 4.55 + 3.35) communities. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Determining the Fraction of Organic Carbon RISC Nondefault Option
Indiana Department of Environmental Management Office of Land Quality 100 N. Senate Indianapolis, IN 46204-2251 GUIDANCE OLQ PH: (317) 232-8941 Indiana Department of Environmental Management Office of Land Quality Determining the Fraction of Organic Carbon RISC Nondefault Option Background Soil can be a complex mixture of mineral-derived compounds and organic matter. The ratios of each component can vary widely depending on the type of soil being investigated. Soil organic matter is a term used by agronomists for the total organic portion of the soil and is derived from decomposed plant matter, microorganisms, and animal residues. The decomposition process can create complex high molecular weight biopolymers (e.g., humic acid) as well as simpler organic compounds (decomposed lignin or cellulose). Only the simpler organic compounds contribute to the fraction of organic carbon. There is not a rigorous definition of the fraction of organic carbon (Foc). However, it can be thought of as the portion of the organic matter that is available to adsorb the organic contaminants of concern. The higher the organic carbon content, the more organic chemicals may be adsorbed to the soil and the less of those chemicals will be available to leach to the ground water. A nondefault option in the Risk Integrated System of Closure (RISC) is to use Foc in the Soil to Ground Water Partitioning Model to calculate a site specific migration to ground water closure level. In the Soil to Ground Water Partition Model, the coefficient, Kd, for organic compounds is the Foc multiplied by the chemical-specific soil organic carbon water partition coefficient, Koc. -

Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O
1122 Biomineralization and Global Biogeochemical Cycles Philippe Van Cappellen Faculty of Geosciences, Utrecht University P.O. Box 80021 3508 TA Utrecht, The Netherlands INTRODUCTION Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere- hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment. Biogeochemical cycles provide a general framework in which geochemists organize their knowledge and interpret their data. The cycle of a given element or substance maps out the rates of transformation in, and transport fluxes between, adjoining environmental reservoirs. The temporal and spatial scales of interest dictate the selection of reservoirs and processes included in the cycle. Typically, the need for a detailed representation of biological process rates and ecosystem structure decreases as the spatial and temporal time scales considered increase. Much progress has been made in the development of global-scale models of biogeochemical cycles. Although these models are based on fairly simple representations of the biosphere and hydrosphere, they account for the large-scale changes in the composition, redox state and biological productivity of the earth surface environment that have occurred over geological time. -

Improved Efficiency of the Biological Pump As a Trigger for the Late Ordovician Glaciation
ARTICLES https://doi.org/10.1038/s41561-018-0141-5 Improved efficiency of the biological pump as a trigger for the Late Ordovician glaciation Jiaheng Shen1,2*, Ann Pearson1*, Gregory A. Henkes1,3, Yi Ge Zhang1,4, Kefan Chen5, Dandan Li5, Scott D. Wankel 6, Stanley C. Finney7 and Yanan Shen 5 The first of the ‘Big Five’ Phanerozoic mass extinctions occurred in tandem with an episode of glaciation during the Hirnantian Age of the Late Ordovician. The mechanism or change in the carbon cycle that promoted this glaciation, thereby resulting in the extinction, is still debated. Here we report new, coupled nitrogen isotope analyses of bulk sediments and chlorophyll degrada- tion products (porphyrins) from the Vinini Creek section (Vinini Formation, Nevada, USA) to show that eukaryotes increas- ingly dominated marine export production in the lead-up to the Hirnantian extinction. We then use these findings to evaluate changes in the carbon cycle by incorporating them into a biogeochemical model in which production is increased in response to an elevated phosphorus inventory, potentially caused by enhanced continental weathering in response to the activity of land plants and/or an episode of volcanism. The results suggest that expanded eukaryotic algal production may have increased the community average cell size, leading to higher export efficiency during the Late Katian. The coincidence of this community shift with a large-scale marine transgression increased organic carbon burial, drawing down CO2 and triggering the Hirnantian glaciation. This episode may mark an early Palaeozoic strengthening of the biological pump, which, for a short while, may have made eukaryotic algae indirect killers. -

A Diurnal Carbon Engine Explains 13C-Enriched Carbonates
A diurnal carbon engine explains 13C-enriched carbonates without increasing the global production of oxygen Emily C. Geymana,1 and Adam C. Maloofa aDepartment of Geosciences, Princeton University, Princeton, NJ 08544 Edited by Donald E. Canfield, Institute of Biology and Nordic Center for Earth Evolution, University of Southern Denmark, Odense M., Denmark, and approved October 8, 2019 (received for review May 21, 2019) In the past 3 billion years, significant volumes of carbonate processes can be constrained by visualizing the carbon evolution with high carbon-isotopic (δ13C) values accumulated on shallow of banktop seawater in a Deffeyes diagram (9) (Fig. 2B), since continental shelves. These deposits frequently are interpreted each mechanism induces a characteristic change in DIC and total as records of elevated global organic carbon burial. However, alkalinity (TA) (Table 1). Observations of TOC (5) and DIC vs. through the stoichiometry of primary production, organic carbon TA (7) from the GBB constrain the relative sizes of the net car- burial releases a proportional amount of O2, predicting unrealis- bonate, gas-exchange, and organic fluxes to be 62, 37, and <1%, tic rises in atmospheric pO2 during the 1 to 100 million year-long respectively (Fig. 2). Although the net burial of organic matter positive δ13C excursions that punctuate the geological record. is close to zero, the flux of carbon between organic matter and This carbon–oxygen paradox assumes that the δ13C of shallow the DIC pool over the course of a single diurnal (24-h) cycle water carbonates reflects the δ13C of global seawater-dissolved can exceed the gross atmospheric and carbonate fluxes by over inorganic carbon (DIC).