Productivity of Forests of the United States and Its Relation to Soil and Site Factors and Management Practices: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Freshwater Ecosystems and Biodiversity
Network of Conservation Educators & Practitioners Freshwater Ecosystems and Biodiversity Author(s): Nathaniel P. Hitt, Lisa K. Bonneau, Kunjuraman V. Jayachandran, and Michael P. Marchetti Source: Lessons in Conservation, Vol. 5, pp. 5-16 Published by: Network of Conservation Educators and Practitioners, Center for Biodiversity and Conservation, American Museum of Natural History Stable URL: ncep.amnh.org/linc/ This article is featured in Lessons in Conservation, the official journal of the Network of Conservation Educators and Practitioners (NCEP). NCEP is a collaborative project of the American Museum of Natural History’s Center for Biodiversity and Conservation (CBC) and a number of institutions and individuals around the world. Lessons in Conservation is designed to introduce NCEP teaching and learning resources (or “modules”) to a broad audience. NCEP modules are designed for undergraduate and professional level education. These modules—and many more on a variety of conservation topics—are available for free download at our website, ncep.amnh.org. To learn more about NCEP, visit our website: ncep.amnh.org. All reproduction or distribution must provide full citation of the original work and provide a copyright notice as follows: “Copyright 2015, by the authors of the material and the Center for Biodiversity and Conservation of the American Museum of Natural History. All rights reserved.” Illustrations obtained from the American Museum of Natural History’s library: images.library.amnh.org/digital/ SYNTHESIS 5 Freshwater Ecosystems and Biodiversity Nathaniel P. Hitt1, Lisa K. Bonneau2, Kunjuraman V. Jayachandran3, and Michael P. Marchetti4 1U.S. Geological Survey, Leetown Science Center, USA, 2Metropolitan Community College-Blue River, USA, 3Kerala Agricultural University, India, 4School of Science, St. -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
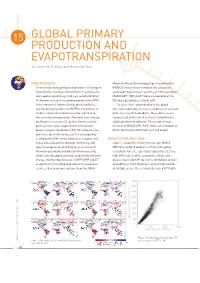
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Microbial Loop' in Stratified Systems
MARINE ECOLOGY PROGRESS SERIES Vol. 59: 1-17, 1990 Published January 11 Mar. Ecol. Prog. Ser. 1 A steady-state analysis of the 'microbial loop' in stratified systems Arnold H. Taylor, Ian Joint Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth PLl 3DH, United Kingdom ABSTRACT. Steady state solutions are presented for a simple model of the surface mixed layer, which contains the components of the 'microbial loop', namely phytoplankton, picophytoplankton, bacterio- plankton, microzooplankton, dissolved organic carbon, detritus, nitrate and ammonia. This system is assumed to be in equilibrium with the larger grazers present at any time, which are represented as an external mortality function. The model also allows for dissolved organic nitrogen consumption by bacteria, and self-grazing and mixotrophy of the microzooplankton. The model steady states are always stable. The solution shows a number of general properties; for example, biomass of each individual component depends only on total nitrogen concentration below the mixed layer, not whether the nitrogen is in the form of nitrate or ammonia. Standing stocks and production rates from the model are compared with summer observations from the Celtic Sea and Porcupine Sea Bight. The agreement is good and suggests that the system is often not far from equilibrium. A sensitivity analysis of the model is included. The effect of varying the mixing across the pycnocline is investigated; more intense mixing results in the large phytoplankton population increasing at the expense of picophytoplankton, micro- zooplankton and DOC. The change from phytoplankton to picophytoplankton dominance at low mixing occurs even though the same physiological parameters are used for both size fractions. -

Relationships Between Net Primary Production, Water Transparency, Chlorophyll A, and Total Phosphorus in Oak Lake, Brookings County, South Dakota
Proceedings of the South Dakota Academy of Science, Vol. 92 (2013) 67 RELATIONSHIPS BETWEEN NET PRIMARY PRODUCTION, WATER TRANSPARENCY, CHLOROPHYLL A, AND TOTAL PHOSPHORUS IN OAK LAKE, BROOKINGS COUNTY, SOUTH DAKOTA Lyntausha C. Kuehl and Nels H. Troelstrup, Jr.* Department of Natural Resource Management South Dakota State University Brookings, SD 57007 *Corresponding author email: [email protected] ABSTRACT Lake trophic state is of primary concern for water resource managers and is used as a measure of water quality and classification for beneficial uses. Secchi transparency, total phosphorus and chlorophyll a are surrogate measurements used in the calculation of trophic state indices (TSI) which classify waters as oligotrophic, mesotrophic, eutrophic or hypereutrophic. Yet the relationships between these surrogate measurements and direct measures of lake productivity vary regionally and may be influenced by external factors such as non-algal tur- bidity. Prairie pothole basins, common throughout eastern South Dakota and southwestern Minnesota, are shallow glacial lakes subject to frequent winds and sediment resuspension. Light-dark oxygen bottle methodology was employed to evaluate vertical planktonic production within an eastern South Dakota pothole basin. Secchi transparency, total phosphorus and planktonic chlorophyll a were also measured from each of three basin sites at biweekly intervals throughout the 2012 growing season. Secchi transparencies ranged between 0.13 and 0.25 meters, corresponding to an average TSISD value of 84.4 (hypereutrophy). Total phosphorus concentrations ranged between 178 and 858 ug/L, corresponding to an average TSITP of 86.7 (hypereutrophy). Chlorophyll a values corresponded to an average TSIChla value of 69.4 (transitional between eutrophy and hypereutro- phy) and vertical production profiles yielded areal net primary productivity val- ues averaging 288.3 mg C∙m-2∙d-1 (mesotrophy). -

Supplement A: Assumptions and Equations in Ecopath with Ecosim Governing Equations
Transactions of the American Fisheries Society 145:136–162, 2016 © American Fisheries Society 2016 DOI: 10.1080/00028487.2015.1069211 Supplement A: Assumptions and Equations in Ecopath with Ecosim Governing Equations The Ecopath module of EwE is a static, mass-balance ecosystem model that uses two governing equations for each species and age group (Christensen and Walters 2004). The first governing equation describes each species group’s production for a time period, i.e., production (P) is the sum of fishery catch (F), predation (M2), net migration (immigration, I, and emigration, E), biomass accumulation (BA), and mortality from other sources (‘other mortality’, M0): P = F + M2 + (I - E) + BA - M0 The second governing equation is based on the principle of conservation of matter within a group, and is designed to balance the energy flows of a biomass pool, i.e., consumption (C) equals the sum of production (P), respiration (R), and unassimilated food (U): C = P + R + U At a minimum, Ecopath requires inputs of diet composition (DCi,j, where i is predator and j is prey), fishery catch (Yi), and three of the following four parameters for each model group (i): biomass (Bi), production-to-biomass ratio (Pi/Bi), consumption-to-biomass ratio (Qi/Bi), and the ecotrophic efficiency (EEi, the fraction of the production that is used in the system and does not move directly to the detritus pool). Mass-balance principles are then used to estimate the fourth parameter. P/B is the annual production rate of the population in Ecopath. Under equilibrium conditions, the P/B ratio of fish is equivalent to its total annual instantaneous mortality (Z) (Allen 1971). -

Productivity Is Defined As the Ratio of Output to Input(S)
Institute for Development Policy and Management (IDPM) Development Economics and Public Policy Working Paper Series WP No. 31/2011 Published by: Development Economics and Public Policy Cluster, Institute of Development Policy and Management, School of Environment and Development, University of Manchester, Manchester M13 9PL, UK; email: [email protected]. PRODUCTIVITY MEASUREMENT IN INDIAN MANUFACTURING: A COMPARISON OF ALTERNATIVE METHODS Vinish Kathuria SJMSOM, Indian Institute of Technology Bombay [email protected] Rajesh S N Raj * Centre for Multi-Disciplinary Development Research, Dharwad [email protected] Kunal Sen IDPM, University of Manchester [email protected] Abstract Very few other issues in explaining economic growth has generated so much debate than the measurement of total factor productivity (TFP) growth. The concept of TFP and its measurement and interpretation have offered a fertile ground for researchers for more than half a century. This paper attempts to provide a review of different issues in the measurement of TFP including the choice of inputs and outputs. The paper then gives a brief review of different techniques used to compute TFP growth. Using three different techniques – growth accounting (non-parametric), production function accounting for endogeniety (semi-parametric) and stochastic production frontier (parametric) – the paper computes the TFP growth of Indian manufacturing for both formal and informal sectors from 1989-90 to 2005-06. The results indicate that the TFP growth of formal and informal sector has differed greatly during this 16-year period but that the estimates are sensitive to the technique used. This suggests that any inference on productivity growth in India since the economic reforms of 1991 is conditional on the method of measurement used, and that there is no unambiguous picture emerging on the direction of change in TFP growth in post-reform India. -

Structure and Distribution of Cold Seep Communities Along the Peruvian Active Margin: Relationship to Geological and Fluid Patterns
MARINE ECOLOGY PROGRESS SERIES Vol. 132: 109-125, 1996 Published February 29 Mar Ecol Prog Ser l Structure and distribution of cold seep communities along the Peruvian active margin: relationship to geological and fluid patterns 'Laboratoire Ecologie Abyssale, DROIEP, IFREMER Centre de Brest, BP 70, F-29280 Plouzane, France '~epartementdes Sciences de la Terre, UBO, 6 ave. Le Gorgeu, F-29287 Brest cedex, France 3~aboratoireEnvironnements Sedimentaires, DROIGM, IFREMER Centre de Brest, BP 70, F-29280 Plouzane, France "niversite P. et M. Curie, Observatoire Oceanologique de Banyuls, F-66650 Banyuls-sur-Mer, France ABSTRACT Exploration of the northern Peruvian subduction zone with the French submersible 'Nau- tile' has revealed benthlc communities dominated by new species of vesicomyid bivalves (Calyptogena spp and Ves~comyasp ) sustained by methane-nch fluid expulsion all along the continental margin, between depths of 5140 and 2630 m Videoscoplc studies of 25 dives ('Nautiperc cruise 1991) allowed us to describe the distribution of these biological conlnlunities at different spahal scales At large scale the communities are associated with fluid expuls~onalong the major tectonic features (scarps, canyons) of the margln At a smaller scale on the scarps, the distribuhon of the communities appears to be con- trolled by fluid expulsion along local fracturatlon features such as joints, faults and small-scale scars Elght dlves were made at one particular geological structure the Middle Slope Scarp (the scar of a large debns avalanche) where numerous -

Phytoplankton Primary Production and Its Utilization by the Pelagic Community in the Coastal Zone of the Gulf of Gdahsk (Southern Baltic)
MARINE ECOLOGY PROGRESS SERIES Vol. 148: 169-186. 1997 Published March 20 Mar Ecol Prog Ser l Phytoplankton primary production and its utilization by the pelagic community in the coastal zone of the Gulf of Gdahsk (southern Baltic) Zbigniew Witekl-*,Stanislaw Ochockil, Modesta ~aciejowska~, Marianna Pastuszakl, Jan ~akonieczny',Beata podgorska2, Janina M. ~ownacka', Tomasz Mackiewiczl, Magdalena Wrzesinska-Kwiecieh' 'Sea Fisheries Institute, ul. Koltqtaja 1, 81-332 Gdynia, Poland 'Marine Biology Center, Polish Academy of Sciences, ul. SW. Wojciecha S,81-347 Gdynia, Poland ABSTRACT In th~sstudy we estimated the amount and fate of phytoplankton primary product~onin the coastal zone of the Gulf of Gdansk, Poland, an area exposed to nutnent enrichment from the Vis- tuld R~verand nearby inunlcipal agglomeration The ~nvestigat~onswere carned out at 2 sites dur~ng 5 months in 1993 (February, April, May, August and October). A prolonged bloom pei-iod occurred in the coastal zone, as compared to the open Gulf and the open sea waters. From Aprll until October most values of gross primary production in the near-surface layer were in the range 100 to 500 mgC m-'' d1 Phytoplankton net exudate release constituted on average 5% of the gross prlmai-y production, total exudate release was estimated to be about 2 times h~gher.Bacterial production in the growth season was relatively low (the mean value ly~ngbetween 5 and 9%of gloss primary production), nevertheless, the microbial community (bactena and protozoans) ut~l~zeda large proportion of primary production (from about 50% in April and May to 16'%, in October). -

Carlson's Trophic State Index
Carlson's Trophic State Index The cloudiness of lake water and how far down you can see is often related to the amount of nutrients in the water. Nutrients promote growth of microscopic plant cells (phytoplankton) that are fed upon by microscopic animals (zooplankton). The more the nutrients, the more the plants and animals and the cloudier the water is. This is a common, but indirect, way to roughly estimate the condition of the lake. This condition, called eutrophication, is a natural aging process of lakes, but which is unnaturally accelerated by too many nutrients. A Secchi disk is commonly used to measure the depth to which you can easily see through the water, also called its transparency. Secchi disk transparency, chlorophyll a (an indirect measure of phytoplankton), and total phosphorus (an important nutrient and potential pollutant) are often used to define the degree of eutrophication, or trophic status of a lake. The concept of trophic status is based on the fact that changes in nutrient levels (measured by total phosphorus) causes changes in algal biomass (measured by chlorophyll a) which in turn causes changes in lake clarity (measured by Secchi disk transparency). A trophic state index is a convenient way to quantify this relationship. One popular index was developed by Dr. Robert Carlson of Kent State University. Trophic State Index Carlson's index uses a log transformation of Secchi disk values as a measure of algal biomass on a scale from 0 - 110. Each increase of ten units on the scale represents a doubling of algal biomass. Because chlorophyll a and total phosphorus are usually closely correlated to Secchi disk measurements, these parameters can also be assigned trophic state index values.