Phytoplankton Primary Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
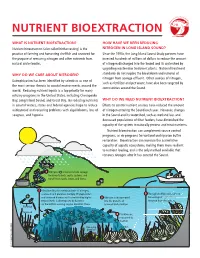
Nutrient Bioextraction L
soun nd d s sla tu i d g y n o NUTRIENT BIOEXTRACTION l WHAT IS NUTRIENT BIOEXTRACTION? HOW HAVE WE BEEN REDUCING Nutrient bioextraction (also called bioharvesting) is the NITROGEN IN LONG ISLAND SOUND? practice of farming and harvesting shellfish and seaweed for Since the 1990s, the Long Island Sound Study partners have the purpose of removing nitrogen and other nutrients from invested hundreds of millions of dollars to reduce the amount natural water bodies. of nitrogen discharged into the Sound and its watershed by upgrading wastewater treatment plants. National treatment WHY DO WE CARE ABOUT NITROGEN? standards do not require the breakdown and removal of nitrogen from sewage effluent. Other sources of nitrogen, Eutrophication has been identified by scientists as one of such as fertilizer and pet waste, have also been targeted by the most serious threats to coastal environments around the communities around the Sound. world. Reducing nutrient inputs is a top priority for many estuary programs in the United States, including Chesapeake Bay, Long Island Sound, and Great Bay. By reducing nutrients WHY DO WE NEED NUTRIENT BIOEXTRACTION? in coastal waters, states and federal agencies hope to reduce Efforts to control nutrient sources have reduced the amount widespread and recurring problems with algal blooms, loss of of nitrogen entering the Sound each year. However, changes seagrass, and hypoxia. in the Sound and its watershed, such as wetland loss and decreased populations of filter feeders, have diminished the capacity of the system to naturally process and treat nutrients. Nutrient bioextraction can complement source control programs, as do programs for wetland and riparian buffer restoration. -

Rapid Assay Replicate
Algae Analysis Report and Data Set Customer ID: 000 Tracking Code: 200014-000 Sample ID: Rapid Assay Replicate: . Customer ID: 000 Sample Date: 4/30/2020 Sample Level: Epi Job ID: 1 Station: Sample Station Sample Depth: 0 System Name: Sample Lake Site: Sample Site Preservative: Glutaraldehyde Report Notes: Sample Report Note Division: Bacillariophyta Taxa ID Genus Species Subspecies Variety Form Morph Structure Relative Concentration 1109 Diatoma tenuis . Vegetative 1.00 1000936 Lindavia intermedia . Vegetative 18.00 9123 Nitzschia palea . Vegetative 1.00 1293 Stephanodiscus niagarae . Vegetative 2.00 Summary for Division ~ Bacillariophyta (4 detail records) Sum Total Bacillariophyta 22.00 Division: Chlorophyta Taxa ID Genus Species Subspecies Variety Form Morph Structure Relative Concentration 2683 *Chlorococcaceae spp . 2-9.9 um Vegetative 1.00 spherical 2491 Schroederia judayi . Vegetative 25.00 Summary for Division ~ Chlorophyta (2 detail records) Sum Total Chlorophyta 26.00 = Identification is Uncertain 200014-000 Monday, January 18, 2021 * = Family Level Identification Phytoplankton - Rapid Assay Page 2 of 13 Division: Cryptophyta Taxa ID Genus Species Subspecies Variety Form Morph Structure Relative Concentration 3015 Cryptomonas erosa . Vegetative 15.00 3043 Rhodomonas minuta . nannoplanctica . Vegetative 2.00 Summary for Division ~ Cryptophyta (2 detail records) Sum Total Cryptophyta 17.00 Division: Cyanophyta Taxa ID Genus Species Subspecies Variety Form Morph Structure Relative Concentration 4041 Aphanizomenon flos-aquae . -

A New Type of Plankton Food Web Functioning in Coastal Waters Revealed by Coupling Monte Carlo Markov Chain Linear Inverse Metho
A new type of plankton food web functioning in coastal waters revealed by coupling Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis Marouan Meddeb, Nathalie Niquil, Boutheina Grami, Kaouther Mejri, Matilda Haraldsson, Aurélie Chaalali, Olivier Pringault, Asma Sakka Hlaili To cite this version: Marouan Meddeb, Nathalie Niquil, Boutheina Grami, Kaouther Mejri, Matilda Haraldsson, et al.. A new type of plankton food web functioning in coastal waters revealed by coupling Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis. Ecological Indicators, Elsevier, 2019, 104, pp.67-85. 10.1016/j.ecolind.2019.04.077. hal-02146355 HAL Id: hal-02146355 https://hal.archives-ouvertes.fr/hal-02146355 Submitted on 3 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 A new type of plankton food web functioning in coastal waters revealed by coupling 2 Monte Carlo Markov Chain Linear Inverse method and Ecological Network Analysis 3 4 5 Marouan Meddeba,b*, Nathalie Niquilc, Boutheïna Gramia,d, Kaouther Mejria,b, Matilda 6 Haraldssonc, Aurélie Chaalalic,e,f, Olivier Pringaultg, Asma Sakka Hlailia,b 7 8 aUniversité de Carthage, Faculté des Sciences de Bizerte, Laboratoire de phytoplanctonologie 9 7021 Zarzouna, Bizerte, Tunisie. -

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

8 April 2021 Mr. Jon Kurland Assistant
8 April 2021 Mr. Jon Kurland Assistant Regional Administrator Protected Resources Division, Alaska Region National Marine Fisheries Service P.O. Box 21668 Juneau, Alaska 99082-1668 Dear Mr. Kurland: The Marine Mammal Commission (the Commission), in consultation with its Committee of Scientific Advisors on Marine Mammals, has reviewed the National Marine Fisheries Service’s (NMFS) 8 January 2021 Federal Register notice (86 Fed. Reg. 1452) revising its proposed designation of critical habitat for Arctic ringed seals (Phoca hispida hispida)1 and reopening the comment period on that proposal. The Commission offers the following comments and recommendations. Background On 28 December 2012, NMFS published a final rule listing the Arctic ringed seal as threatened under the Endangered Species Act (ESA) (77 Fed. Reg. 76706) and requesting information on physical and biological features essential to the conservation of Arctic ringed seals and on economic consequences of designating critical habitat for this species. Section 3(5)(A) of the ESA defines “critical habitat” as: (i) the specific areas within the geographical area occupied by the species, at the time it is listed in accordance with section 4 of this Act, on which are found those physical or biological features (I) essential to the conservation of the species and (II) which may require special management considerations or protection; and (ii) specific areas outside the geographical area occupied by the species at the time it is listed in accordance with the provisions of section 4 of this Act, upon a determination by the Secretary that such areas are essential for the conservation of the species. -

"Phylum" for "Division" for Groups Treated As Plants Author(S): H
Proposal (10) to Substitute the Term "Phylum" for "Division" for Groups Treated as Plants Author(s): H. C. Bold, A. Cronquist, C. Jeffrey, L. A. S. Johnson, L. Margulis, H. Merxmüller, P. H. Raven and A. L. Takhtajan Reviewed work(s): Source: Taxon, Vol. 27, No. 1 (Feb., 1978), pp. 121-122 Published by: International Association for Plant Taxonomy (IAPT) Stable URL: http://www.jstor.org/stable/1220504 . Accessed: 15/08/2012 04:49 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. International Association for Plant Taxonomy (IAPT) is collaborating with JSTOR to digitize, preserve and extend access to Taxon. http://www.jstor.org TAXON 27 (1): 121-132 FEBRUARY 1978 NOMENCLATURE TYPIFICATION OF MICROORGANISMS: A PROPOSAL Ralph A. Lewin * & G. E. Fogg** Rules and recommendations for the typification of many kinds of microorganisms, in- cluding microscopic algae and fungi, need to be updated for a variety of reasons. Improve- ments of fixation techniques normally make it unnecessary to invoke Article 9, Note 1, which relates to "the name of a species of infraspecific taxon of Recent plants of which it is impossible to preserve a specimen..." On the other hand, more and more taxonomy is being based on electron-microscopy, biochemistry, serology or other modern techniques for which living material may be needed, and for which a herbarium sheet is generally inadequate and a drawing, or even a photomicrograph, is for various reasons unsatisfactory. -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Periodic and Coordinated Gene Expression Between a Diazotroph and Its Diatom Host
The ISME Journal (2019) 13:118–131 https://doi.org/10.1038/s41396-018-0262-2 ARTICLE Periodic and coordinated gene expression between a diazotroph and its diatom host 1 1,2 1 3 4 Matthew J. Harke ● Kyle R. Frischkorn ● Sheean T. Haley ● Frank O. Aylward ● Jonathan P. Zehr ● Sonya T. Dyhrman1,2 Received: 11 April 2018 / Revised: 28 June 2018 / Accepted: 28 July 2018 / Published online: 16 August 2018 © International Society for Microbial Ecology 2018 Abstract In the surface ocean, light fuels photosynthetic carbon fixation of phytoplankton, playing a critical role in ecosystem processes including carbon export to the deep sea. In oligotrophic oceans, diatom–diazotroph associations (DDAs) play a keystone role in ecosystem function because diazotrophs can provide otherwise scarce biologically available nitrogen to the diatom host, fueling growth and subsequent carbon sequestration. Despite their importance, relatively little is known about the nature of these associations in situ. Here we used metatranscriptomic sequencing of surface samples from the North Pacific Subtropical Gyre (NPSG) to reconstruct patterns of gene expression for the diazotrophic symbiont Richelia and we – 1234567890();,: 1234567890();,: examined how these patterns were integrated with those of the diatom host over day night transitions. Richelia exhibited significant diel signals for genes related to photosynthesis, N2 fixation, and resource acquisition, among other processes. N2 fixation genes were significantly co-expressed with host nitrogen uptake and metabolism, as well as potential genes involved in carbon transport, which may underpin the exchange of nitrogen and carbon within this association. Patterns of expression suggested cell division was integrated between the host and symbiont across the diel cycle. -

Kelp Cultivation Effectively Improves Water Quality and Regulates Phytoplankton Community in a Turbid, Highly Eutrophic Bay
Science of the Total Environment 707 (2020) 135561 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Kelp cultivation effectively improves water quality and regulates phytoplankton community in a turbid, highly eutrophic bay Zhibing Jiang a,b,d, Jingjing Liu a,ShangluLic,YueChena,PingDua,b,YuanliZhua,YiboLiaoa,d, Quanzhen Chen a, Lu Shou a, Xiaojun Yan e, Jiangning Zeng a,⁎, Jianfang Chen a,d a Key Laboratory of Marine Ecosystem and Biogeochemistry, State Oceanic Administration & Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China b Function Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China c Marine Monitoring and Forecasting Center of Zhejiang Province, Hangzhou, China d State Key Laboratory of Satellite Ocean Environment Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China e Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Marine College of Ningbo University, Ningbo, China HIGHLIGHTS GRAPHICAL ABSTRACT • Kelp farming alleviated eutrophication and acidification. • Kelp farming greatly relieved light limi- tation and increased phytoplankton bio- mass. • Kelp farming appreciably enhanced phytoplankton diversity. • Kelp farming reduced the dominance of dinoflagellate Prorocentrum minimum. • Phytoplankton community differed sig- nificantly between the kelp farm and control area. article info abstract Article history: Coastal eutrophication and its associated harmful algal blooms have emerged as one of the most severe environ- Received 19 September 2019 mental problems worldwide. Seaweed cultivation has been widely encouraged to control eutrophication and Received in revised form 11 November 2019 algal blooms. Among them, cultivated kelp (Saccharina japonica) dominates primarily by production and area. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward.