Lakes, Primary Production, Budgets and Cycling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

7.014 Handout PRODUCTIVITY: the “METABOLISM” of ECOSYSTEMS
7.014 Handout PRODUCTIVITY: THE “METABOLISM” OF ECOSYSTEMS Ecologists use the term “productivity” to refer to the process through which an assemblage of organisms (e.g. a trophic level or ecosystem assimilates carbon. Primary producers (autotrophs) do this through photosynthesis; Secondary producers (heterotrophs) do it through the assimilation of the organic carbon in their food. Remember that all organic carbon in the food web is ultimately derived from primary production. DEFINITIONS Primary Productivity: Rate of conversion of CO2 to organic carbon (photosynthesis) per unit surface area of the earth, expressed either in terns of weight of carbon, or the equivalent calories e.g., g C m-2 year-1 Kcal m-2 year-1 Primary Production: Same as primary productivity, but usually expressed for a whole ecosystem e.g., tons year-1 for a lake, cornfield, forest, etc. NET vs. GROSS: For plants: Some of the organic carbon generated in plants through photosynthesis (using solar energy) is oxidized back to CO2 (releasing energy) through the respiration of the plants – RA. Gross Primary Production: (GPP) = Total amount of CO2 reduced to organic carbon by the plants per unit time Autotrophic Respiration: (RA) = Total amount of organic carbon that is respired (oxidized to CO2) by plants per unit time Net Primary Production (NPP) = GPP – RA The amount of organic carbon produced by plants that is not consumed by their own respiration. It is the increase in the plant biomass in the absence of herbivores. For an entire ecosystem: Some of the NPP of the plants is consumed (and respired) by herbivores and decomposers and oxidized back to CO2 (RH). -

Bacterial Production and Respiration
Organic matter production % 0 Dissolved Particulate 5 > Organic Organic Matter Matter Heterotrophic Bacterial Grazing Growth ~1-10% of net organic DOM does not matter What happens to the 90-99% of sink, but can be production is physically exported to organic matter production that does deep sea not get exported as particles? transported Export •Labile DOC turnover over time scales of hours to days. •Semi-labile DOC turnover on time scales of weeks to months. •Refractory DOC cycles over on time scales ranging from decadal to multi- decadal…perhaps longer •So what consumes labile and semi-labile DOC? How much carbon passes through the microbial loop? Phytoplankton Heterotrophic bacteria ?? Dissolved organic Herbivores ?? matter Higher trophic levels Protozoa (zooplankton, fish, etc.) ?? • Very difficult to directly measure the flux of carbon from primary producers into the microbial loop. – The microbial loop is mostly run on labile (recently produced organic matter) - - very low concentrations (nM) turning over rapidly against a high background pool (µM). – Unclear exactly which types of organic compounds support bacterial growth. Bacterial Production •Step 1: Determine how much carbon is consumed by bacteria for production of new biomass. •Bacterial production (BP) is the rate that bacterial biomass is created. It represents the amount of Heterotrophic material that is transformed from a nonliving pool bacteria (DOC) to a living pool (bacterial biomass). •Mathematically P = µB ?? µ = specific growth rate (time-1) B = bacterial biomass (mg C L-1) P= bacterial production (mg C L-1 d-1) Dissolved organic •Note that µ = P/B matter •Thus, P has units of mg C L-1 d-1 Bacterial production provides one measurement of carbon flow into the microbial loop How doe we measure bacterial production? Production (∆ biomass/time) (mg C L-1 d-1) • 3H-thymidine • 3H or 14C-leucine Note: these are NOT direct measures of biomass production (i.e. -
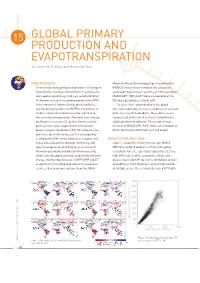
GLOBAL PRIMARY PRODUCTION and EVAPOTRANSPIRATION by Steven W
15 GLOBAL PRIMARY PRODUCTION AND EVAPOTRANSPIRATION by Steven W. Running and Maosheng Zhao DATA PRODUCTS Moderate Resolution Imaging Spectroradiometer Terrestrial primary production provides the energy to (MODIS) sensor, these variables are calculated maintain the structure and functions of ecosystems, every eight days in near real time at 1 km resolution. and supplies goods (e.g. food, fuel, wood and fi bre) MODIS GPP, NPP and ET data are available at the for human society. Gross primary production (GPP) EOS data gateway (see link below). is the amount of carbon fi xed by photosynthesis, To correct for contamination in the global and net primary production (NPP) is the amount of refl ectance data due to severe cloudiness or aerosols carbon converted into biomass after subtracting in the near real time products, these datasets are the cost of plant respiration. The water loss through reprocessed at the end of each year to build more exchange of trace gas CO2 by leaf stomata during stable, permanent datasets. These end-of-year photosynthesis plus evaporation from soil and versions of MODIS GPP, NPP and ET are available at plants is evapotranspiration (ET). ET computes the NTSG, University of Montana (see link below). water lost by a land surface, so it is consequently a component of the water balance in a region, and RESULTS FOR 2000–2006 is therefore relevant for drought monitoring and Figure 1 shows the seven-year average MODIS water management, providing an assessment of NPP for vegetated land on earth at 1 km spatial the water potentially available for human society. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Lake Ecology
Fundamentals of Limnology Oxygen, Temperature and Lake Stratification Prereqs: Students should have reviewed the importance of Oxygen and Carbon Dioxide in Aquatic Systems Students should have reviewed the video tape on the calibration and use of a YSI oxygen meter. Students should have a basic knowledge of pH and how to use a pH meter. Safety: This module includes field work in boats on Raystown Lake. On average, there is a death due to drowning on Raystown Lake every two years due to careless boating activities. You will very strongly decrease the risk of accident when you obey the following rules: 1. All participants in this field exercise will wear Coast Guard certified PFDs. (No exceptions for teachers or staff). 2. There is no "horseplay" allowed on boats. This includes throwing objects, splashing others, rocking boats, erratic operation of boats or unnecessary navigational detours. 3. Obey all boating regulations, especially, no wake zone markers 4. No swimming from boats 5. Keep all hands and sampling equipment inside of boats while the boats are moving. 6. Whenever possible, hold sampling equipment inside of the boats rather than over the water. We have no desire to donate sampling gear to the bottom of the lake. 7. The program director has final say as to what is and is not appropriate safety behavior. Failure to comply with the safety guidelines and the program director's requests will result in expulsion from the program and loss of Field Station privileges. I. Introduction to Aquatic Environments Water covers 75% of the Earth's surface. We divide that water into three types based on the salinity, the concentration of dissolved salts in the water. -

Article Is Available Gen Availability
Hydrol. Earth Syst. Sci., 23, 1763–1777, 2019 https://doi.org/10.5194/hess-23-1763-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Oxycline oscillations induced by internal waves in deep Lake Iseo Giulia Valerio1, Marco Pilotti1,4, Maximilian Peter Lau2,3, and Michael Hupfer2 1DICATAM, Università degli Studi di Brescia, via Branze 43, 25123 Brescia, Italy 2Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Müggelseedamm 310, 12587 Berlin, Germany 3Université du Quebec à Montréal (UQAM), Department of Biological Sciences, Montréal, QC H2X 3Y7, Canada 4Civil & Environmental Engineering Department, Tufts University, Medford, MA 02155, USA Correspondence: Giulia Valerio ([email protected]) Received: 22 October 2018 – Discussion started: 23 October 2018 Revised: 20 February 2019 – Accepted: 12 March 2019 – Published: 2 April 2019 Abstract. Lake Iseo is undergoing a dramatic deoxygena- 1 Introduction tion of the hypolimnion, representing an emblematic exam- ple among the deep lakes of the pre-alpine area that are, to a different extent, undergoing reduced deep-water mixing. In Physical processes occurring at the sediment–water inter- the anoxic deep waters, the release and accumulation of re- face of lakes crucially control the fluxes of chemical com- duced substances and phosphorus from the sediments are a pounds across this boundary (Imboden and Wuest, 1995) major concern. Because the hydrodynamics of this lake was with severe implications for water quality. In stratified shown to be dominated by internal waves, in this study we in- lakes, the boundary-layer turbulence is primarily caused by vestigated, for the first time, the role of these oscillatory mo- wind-driven internal wave motions (Imberger, 1998). -

Phytoplankton Primary Production and Its Utilization by the Pelagic Community in the Coastal Zone of the Gulf of Gdahsk (Southern Baltic)
MARINE ECOLOGY PROGRESS SERIES Vol. 148: 169-186. 1997 Published March 20 Mar Ecol Prog Ser l Phytoplankton primary production and its utilization by the pelagic community in the coastal zone of the Gulf of Gdahsk (southern Baltic) Zbigniew Witekl-*,Stanislaw Ochockil, Modesta ~aciejowska~, Marianna Pastuszakl, Jan ~akonieczny',Beata podgorska2, Janina M. ~ownacka', Tomasz Mackiewiczl, Magdalena Wrzesinska-Kwiecieh' 'Sea Fisheries Institute, ul. Koltqtaja 1, 81-332 Gdynia, Poland 'Marine Biology Center, Polish Academy of Sciences, ul. SW. Wojciecha S,81-347 Gdynia, Poland ABSTRACT In th~sstudy we estimated the amount and fate of phytoplankton primary product~onin the coastal zone of the Gulf of Gdansk, Poland, an area exposed to nutnent enrichment from the Vis- tuld R~verand nearby inunlcipal agglomeration The ~nvestigat~onswere carned out at 2 sites dur~ng 5 months in 1993 (February, April, May, August and October). A prolonged bloom pei-iod occurred in the coastal zone, as compared to the open Gulf and the open sea waters. From Aprll until October most values of gross primary production in the near-surface layer were in the range 100 to 500 mgC m-'' d1 Phytoplankton net exudate release constituted on average 5% of the gross prlmai-y production, total exudate release was estimated to be about 2 times h~gher.Bacterial production in the growth season was relatively low (the mean value ly~ngbetween 5 and 9%of gloss primary production), nevertheless, the microbial community (bactena and protozoans) ut~l~zeda large proportion of primary production (from about 50% in April and May to 16'%, in October). -

A Lake and Pond Classification System for the Northeast and Mid-Atlantic States
A Lake and Pond Classification System for the Northeast and Mid-Atlantic States November 2014 Mark Anderson, Arlene Olivero Sheldon, Alex Jospe The Nature Conservancy Eastern Regional Office Call Outline • Goal • Process • Key Variables – Trophic Level – Alkalinity – Temperature – Depth • Integration of Variables into Types • Distributable Information • Web Mapping Service Goal: A classification and map of waterbodies in the Northeast Product is not intended to override existing state or regional classifications, but is meant to complement and build upon existing classifications to create a seemless eastern U.S. aquatic classification that will provide a means for looking at patterns across the region. Counterpart to the NE Aquatic Habitat Classification Streams and • Lakes and Ponds Rivers Olivero, A, and M.G. Anderson. 2008. The Northeast Aquatic Habitat Classification. The Nature Conservancy, Eastern Conservation Science. 90 pp. http://www.rcngrants.org/spatialData Steering Committee State/Federal Name Agency EPA Jeff Hollister Environmental Protection Agency ME Dave Halliwell Department of Environmental Protection Develop Douglas Suitor Department of Environmental Protection frame- Linda Bacon Department of Environmental Protection Dave Coutemanch The Nature Conservancy work NH Matt Carpenter Division of Fish and Game VT Kellie Merrell Department of Environmental Conservation MA Richard Hartley Department of Fish and Game Share Mark Mattson Department of Environmental Protection CT Brian Eltz DEEP Inland Fisheries Division data NY -

Diurnal and Seasonal Variations of Thermal Stratification and Vertical
Journal of Meteorological Research 1 Yang, Y. C., Y. W. Wang, Z. Zhang, et al., 2018: Diurnal and seasonal variations of 2 thermal stratification and vertical mixing in a shallow fresh water lake. J. Meteor. 3 Res., 32(x), XXX-XXX, doi: 10.1007/s13351-018-7099-5.(in press) 4 5 Diurnal and Seasonal Variations of Thermal 6 Stratification and Vertical Mixing in a Shallow 7 Fresh Water Lake 8 9 Yichen YANG 1, 2, Yongwei WANG 1, 3*, Zhen ZHANG 1, 4, Wei WANG 1, 4, Xia REN 1, 3, 10 Yaqi GAO 1, 3, Shoudong LIU 1, 4, and Xuhui LEE 1, 5 11 1 Yale-NUIST Center on Atmospheric Environment, Nanjing University of Information, 12 Science and Technology, Nanjing 210044, China 13 2 School of Environmental Science and Engineering, Nanjing University of Information, 14 Science and Technology, Nanjing 210044, China 15 3 School of Atmospheric Physics, Nanjing University of Information, Science and Technology, 16 Nanjing 210044, China 17 4 School of Applied Meteorology, Nanjing University of Information, Science and 18 Technology, Nanjing 210044, China 19 5 School of Forestry and Environmental Studies, Yale University, New Haven, CT 06511, 20 USA 21 (Received June 21, 2017; in final form November 18, 2017) 22 23 Supported by the National Natural Science Foundation of China (41275024, 41575147, Journal of Meteorological Research 24 41505005, and 41475141), the Natural Science Foundation of Jiangsu Province, China 25 (BK20150900), the Startup Foundation for Introducing Talent of Nanjing University of 26 Information Science and Technology (2014r046), the Ministry of Education of China under 27 grant PCSIRT and the Priority Academic Program Development of Jiangsu Higher Education 28 Institutions. -

Sinking Jelly-Carbon Unveils Potential Environmental Variability Along a Continental Margin
Sinking Jelly-Carbon Unveils Potential Environmental Variability along a Continental Margin Mario Lebrato1,2*, Juan-Carlos Molinero1, Joan E. Cartes3, Domingo Lloris3, Fre´de´ric Me´lin4, Laia Beni- Casadella3 1 Department of Biogeochemistry and Ecology, Helmholtz Centre for Ocean Research Kiel (GEOMAR), Kiel, Germany, 2 Department of Geosciences, Scripps Institution of Oceanography, San Diego, California, United States of America, 3 Institut de Cie`ncies del Mar de Barcelona (CSIC), Barcelona, Spain, 4 Joint Research Centre, Ispra, Italy Abstract Particulate matter export fuels benthic ecosystems in continental margins and the deep sea, removing carbon from the upper ocean. Gelatinous zooplankton biomass provides a fast carbon vector that has been poorly studied. Observational data of a large-scale benthic trawling survey from 1994 to 2005 provided a unique opportunity to quantify jelly-carbon along an entire continental margin in the Mediterranean Sea and to assess potential links with biological and physical variables. Biomass depositions were sampled in shelves, slopes and canyons with peaks above 1000 carcasses per trawl, translating to standing stock values between 0.3 and 1.4 mg C m2 after trawling and integrating between 30,000 and 175,000 m2 of seabed. The benthopelagic jelly-carbon spatial distribution from the shelf to the canyons may be explained by atmospheric forcing related with NAO events and dense shelf water cascading, which are both known from the open Mediterranean. Over the decadal scale, we show that the jelly-carbon depositions temporal variability paralleled hydroclimate modifications, and that the enhanced jelly-carbon deposits are connected to a temperature-driven system where chlorophyll plays a minor role. -

Lecture 13 - Primary Production: Water Column Processes Prof
12.742 - Marine Chemistry Fall 2004 Lecture 13 - Primary Production: Water Column Processes Prof. Scott Doney Somewhat different organization from years past - start with surface productivity and work • our way down the water column Try to fold methods in as we go • Electronic notes still missing many of the figures - also somewhat different order. • Primary Production – Formation and respiration of organic matter Photosynthesis / respiration – Formation and dissolution of CaCO3, SiO3 Remineralization – Transport of dust, resuspended sediments, Dom Export particles – Scavenging onto particles Sinking Export – Elemental stoichiometry – Water column distributions – Rates Figure 1. hv Phototrophy – Categorize organisms by source of energy, inorganic electron donor and carbon source. CO + H O C + O 2 2 org 2 Chemo litho autotrophy · Chemo organo heterotrophy · Respiration Photo litho autotrophy Q (Chemical Energy) · Figure 2. 1 N N Light absorbed by pigments. • M – mainly chlorophyll a N N – also accessory pigments (which absorb different wavelengths) including forms of chlorophyll (b, c1, c2) carotenoids, biliopro- teins Figure 3. – Chlorophyll has conjugated double bonds - non-localized π orbital electrons, can absorb solar radiation, bump up to higher electron state – PAR - photosynthetic available radiation (350 700 nm) − – Convert light energy into electron energy 400 λ (nm) 700 – Light harvesting pigment-protein complexes Absorb in Blue and Red (leave Green) involved in electron transfer, funneling ex- cited electrons to reaction sites. -

Ocean Primary Production
Learning Ocean Science through Ocean Exploration Section 6 Ocean Primary Production Photosynthesis very ecosystem requires an input of energy. The Esource varies with the system. In the majority of ocean ecosystems the source of energy is sunlight that drives photosynthesis done by micro- (phytoplankton) or macro- (seaweeds) algae, green plants, or photosynthetic blue-green or purple bacteria. These organisms produce ecosystem food that supports the food chain, hence they are referred to as primary producers. The balanced equation for photosynthesis that is correct, but seldom used, is 6CO2 + 12H2O = C6H12O6 + 6H2O + 6O2. Water appears on both sides of the equation because the water molecule is split, and new water molecules are made in the process. When the correct equation for photosynthe- sis is used, it is easier to see the similarities with chemo- synthesis in which water is also a product. Systems Lacking There are some ecosystems that depend on primary Primary Producers production from other ecosystems. Many streams have few primary producers and are dependent on the leaves from surrounding forests as a source of food that supports the stream food chain. Snow fields in the high mountains and sand dunes in the desert depend on food blown in from areas that support primary production. The oceans below the photic zone are a vast space, largely dependent on food from photosynthetic primary producers living in the sunlit waters above. Food sinks to the bottom in the form of dead organisms and bacteria. It is as small as marine snow—tiny clumps of bacteria and decomposing microalgae—and as large as an occasional bonanza—a dead whale.