Administration of Oil-Based Depot and Long-Acting IM Antipsychotic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lipid-Based Depots: Manufacturing, Administration and Interactions of Protein Drugs with Lipid Formulations
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Lipid-based depots: manufacturing, administration and interactions of protein drugs with lipid formulations Michaela Maria Breitsamer aus Starnberg, Deutschland 2019 II ERKLÄRUNG Diese Dissertation wurde im Sinne von § 7 der Promotionsordnung vom 28. November 2011 von Herrn Prof. Dr. Gerhard Winter betreut. EIDESSTATTLICHE VERSICHERUNG Diese Dissertation wurde eigenständig und ohne unerlaubte Hilfe erarbeitet. Wolfratshausen, den 20.05.2019 ________________________________ (Michaela Breitsamer) Dissertation eingereicht am: 20.05.2019 1. Gutachter: Prof. Dr. Gerhard Winter 2. Gutachter: Prof. Dr. Wolfgang Frieß Mündliche Prüfung am: 25.06.2019 III IV FOR MY FAMILY “The way to get started is to quit talking and begin doing” Walt Disney (1901 – 1966) V VI ACKNOWLEDGEMENTS The present thesis was prepared between March 2015 and December 2018 at the Department of Pharmacy, Pharmaceutical Technology and Biopharmaceutics at the Ludwig-Maximilians Universität (LMU) in Munich under the supervision of Prof. Dr. Gerhard Winter. First of all, I would like to express my deepest gratitude to my supervisor Prof. Dr. Gerhard Winter for giving me the opportunity to join his research group and to work on this extremely interesting and interdisciplinary project. I really appreciated his scientific input throughout all phases of this work, his elaborate advice and his guidance, which also contributed to my personal development over the last years. Furthermore, I would like to thank him for the outstanding working and team atmosphere, which he created at the chair, for supporting my participation in scientific conferences and for initiating and motivating me to the numerous collaborations which contributed to a successful thesis. -

Food and Drugs Regulations
LAWS OF TRINIDAD AND TOBAGO 22 Chap. 30:01 Food and Drugs SUBSIDIARY LEGISLATION FOOD AND DRUGS REGULATIONS ARRANGEMENT OF REGULATIONS REGULATION 1. Citation. 2. Requirements prescribed by regulation. 3. Interpretation. 4. Request to Director. 5. Functions, duties, responsibilities of Inspectors. 6. Certificate of appointment. 7. Taking of photographs. 8. Taking samples and detention pending further examination. 9. Violation of Act or Regulations and relabelling or recondition ing of food, drug, cosmetic or device. 10. Issue of certificate. 11. Taking a sample, notification of intention and division of sample. 12. Division an interference and objection to procedure by owner or person. 13. Certificate of Analysis. 14. Definition of terms in Part II. 15. Offence to sell unlabelled food. 16. Labelling of package. 17. Declaration of net contents not required on certain labels. 18. List of ingredients not required on certain labels. 19. Declaration not required. 20. Declaration not required to indicate presence of flavouring. 21. Dried or dehydrated products. 22. Food from vending machine. 23. Non application of regulation 16. 24. Standard for a food. 25. Name of designation given to standard, grade or definition. 26. Adulteration of food. 27. Non-adulteration. 28. Contents of package. 29. Display of information on label. 30. First Schedule. 31. Offence. 32. Definition of terms in Part III. 33. Labelling of drug. 34. Contents of label. 35. Label on bulk package. 36. Drug sold on prescription. 37. Packing cases. LAWS OF TRINIDAD AND TOBAGO Food and Drugs Chap. 30:01 23 Food and Drugs Regulations [Subsidiary} REGULATION 38. Name and proportion of drug to be stated on label. -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -
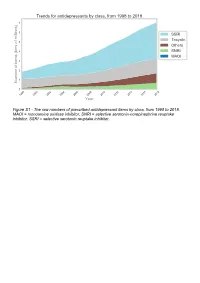
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Depot Antipsychotic Injections
Mental Health Services Good Practice Statement For The Use Of DEPOT & LONG ACTING ANTIPSYCHOTIC INJECTIONS Date of Revision: February 2020 Approved by: Mental Health Prescribing Management Group Review Date: CONSULTATION The document was sent to Community Mental Health teams, Pharmacy and the Psychiatric Advisory Committee for comments prior to completion. KEY DOCUMENTS When reading this policy please consult the NHS Consent Policy when considering any aspect of consent National Institute for Clinical Excellence CG178 (2014), Psychosis and schizophrenia in adults: prevention and management NHS Lothian (2011), Depot Antipsychotic Guidance, Maudsley (2015), Prescribing Guidelines in Psychiatry 12th edition. UKPPG (2009) Guidance on the Administration to Adults of Oil based Depot and other Long Acting Intramuscular Antipsychotic Injections Summary of Product Characteristics for: Clopixol (Zuclopethixol Decanoate) Flupenthixol Decanoate Haldol (Haloperidol Decanoate) Risperdal Consta Xeplion (Paliperidone Palmitate) ZypAdhera (Olanzapine Embonate) Abilify Maintena (Aripiprazole) 1 CONTENTS REFERENCES/KEY DOCUMENTS …………………………………………………………… 1 INTRODUCTION …………………………………………………………………………………. 3 SCOPE …………………………………………………………………………………………….. 3 DEFINITIONS ……………………………………………………………………………………... 3 CHOICE OF DRUG AND DOSAGE SELECTION ……………………………………………. 3 SIDE EFFECTS ……………………………………………………………….………………….. 5 PRESCRIBING …………………………………………………………………………………… 5 ADMINISTRATION ………………………………………………………………………………. 7 RECORDING ADMINISTRATION ……………………………………………………………… 7 -

Deanxit SPC.Pdf
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deanxit film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Flupentixol 0.5 mg (as 0.584 mg flupentixol dihydrochloride) Melitracen 10 mg (as 11.25 mg melitracen hydrochloride) Excipients with known effect: Lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablets. Round, biconvex, cyclamen, film-coated tablets. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Anxiety Depression Asthenia. Neurasthenia. Psychogenic depression. Depressive neuroses. Masked depression. Psychosomatic affections accompanied by anxiety and apathy. Menopausal depressions. Dysphoria and depression in alcoholics and drug-addicts. 4.2 Posology and method of administration Posology Adults Usually 2 tablets daily: morning and noon. In severe cases the morning dose may be increased to 2 tablets. The maximum dose is 4 tablets daily. Older people (> 65 years) 1 tablet in the morning. In severe cases 1 tablet in the morning and 1 at noon. Maintenance dose: Usually 1 tablet in the morning. SPC Portrait-REG_00051968 20v038 Page 1 of 10 In cases of insomnia or severe restlessness additional treatment with a sedative in the acute phase is recommended. Paediatric population Children and adolescents (<18 years) Deanxit is not recommended for use in children and adolescents due to lack of data on safety and efficacy. Reduced renal function Deanxit can be given in the recommended doses. Reduced liver function Deanxit can be given in the recommended doses. Method of administration The tablets are swallowed with water. 4.3 Contraindications Hypersensitivity to flupentixol and melitracen or to any of the excipients listed in section 6.1. -

North of England Guidance for Long Acting Antipsychotic Injections July 17
Guidance on the Use of Antipsychotic Long-acting Injections in North of England (TEWV version) This guidance aims to inform and support prescribers within the three mental health service providers in the north of England in the cost-effective use of antipsychotic long-acting injections. Long-acting or “depot” injections are a useful and well-established form of administering antipsychotics in the management of schizophrenia and other psychoses. The introduction of long-acting formulations of second-generation (atypical) antipsychotics has created an additional pressure on drug budgets in mental health services, which may be justified if their use results in reduced hospital admissions, shortened length of stay and improved quality of life compared with the first generation (typical) agents. Advantages of long-acting / depot injections Assured compliance with antipsychotic treatment Increased bioavailability (less first-pass metabolism) Steady plasma levels compared to oral medication Reduction in relapse rate, severity of relapse and rehospitalisation Stable therapeutic effects Better downward titration to minimise side-effects There is some evidence that long-acting injections cause less brain tissue loss and deterioration (CATIE study1) Disadvantages of long-acting / depot injections Treatment cannot be stopped quickly if severe side-effects develop (dystonia, EPSE, NMS) Perception by the patient of “being controlled”, losing control over their treatment, or possibly being punished. Pain at the site of injection, lasting possibly 10 days Tissue necrosis - over time hard plaques may form, which will reduce the ease of administration and the efficacy of the injection as well as causing discomfort. Loss of dignity with the gluteal route Over and above these factors, NICE2 recommend that consideration should be given to offering a depot / long-acting injectable antipsychotic to people with psychosis or schizophrenia who express a preference for such treatment after an acute episode. -

Formulary Update
PEI Drug Programs Formulary Update Issue 06-04 October, 2006 The following changes and additions to the July 2006 edition of the PEI Drug Programs Formulary will be effective October 16th, 2006 unless otherwise noted within this update. If there are any questions regarding the information presented in this update please call the Drug Program office at 368-4947 or toll free at 1-877-577-3737. 1.0 Changes and Additions To The Formulary & MAC Pricing ALMOTRIPTAN (new addition) EDS Criteria: For the treatment of migraine headaches where other standard therapies, such as analgesics and/or ergotamine products have failed. Eligibility is restricted to persons over the age of 18 and under 65 years of age. Coverage is limited to 6 tablets per 30 day period. Persons requiring more than 6 doses per 30 day period should be considered for migraine prophylaxis therapy if they are not already receiving such therapy. 6.25mg tablet 02248128 AXERT (EDS) JAN FW 12.5mg tablet 02248129 AXERT (EDS) JAN FW DILTIAZEM HYDROCHLORIDE (new addition) 120mg capsule 02231150 TIAZAC BVL FNSW 180mg capsule 02231151 TIAZAC BVL FNSW 240mg capsule 02231152 TIAZAC BVL FNSW 300mg capsule 02231154 TIAZAC BVL FNSW 360mg capsule 02231155 TIAZAC BVL FNSW PEI Drug Programs Formulary Update Issue 06-04 October 2006 Page 1 DILTIAZEM HYDROCHLORIDE (new addition) 120mg extended release tablet 02256738 TIAZAC XC BVL FNSW 180mg extended release tablet 02256746 TIAZAC XC BVL FNSW 240mg extended release tablet 02256754 TIAZAC XC BVL FNSW 300mg extended release tablet 02256762 TIAZAC XC BVL FNSW 360mg extended release tablet 02256770 TIAZAC XC BVL FNSW ETHINYL ESTRADIOL/DROSPIRENONE (new addition) 3.0mg/0.03mg tablets 02261723 YASMIN 21 DAY BEX FW 02261731 YASMIN 28 DAY BEX FW ESPROSARTAN MESYLATE & HYDROCHLOROTHIAZIDE (new addition) 600mg & 12.5mg tablet 02253631 TEVETEN PLUS SLV FNSW GABAPENTIN (change) There is no longer an EDS restriction on this drug. -

020592Orig1s040s041
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 020592Orig1s040s041 MEDICAL REVIEW(S) M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: July 18, 2008 FROM: Ni A. Khin, M.D. Team Leader Division of Psychiatry Products, HFD-130 TO: NDA 20-592/SE5-040 (bipolar I disorder, acute mania) NDA 20-592/SE5-041 (schizophrenia) (This overview should be filed with the 02-05-2008 submission in response to the Agency’s Approvable Letter dated 04-30-2007) SUBJECT: Recommendation of an approvable action for use of Zyprexa (olanzapine) in the treatment of 1) Bipolar I disorder, Mania, and 2) schizophrenia in Adolescents. 1. BACKGROUND Zyprexa (olanzapine) is an atypical antipsychotic agent, approved in the U.S. for treatment of schizophrenia and bipolar disorder, mania or mixed episodes, as monotherapy (both acute and maintenance) or combination therapy in adults. It is available as oral 2.5, 5, 10, 15, or 20 mg strength tablets; 5, 10, 15, or 20 mg oral disintegrating tablets (Zydis). The target dose for adults with schizophrenia is 10 mg/day. Zyprexa intramuscular injection (10 mg) is indicated for agitation associated with schizophrenia and Bipolar I Mania. Currently, two atypical antipsychotic drugs, Risperdal and Abilify, are approved for treatment of schizophrenia and bipolar disorder in the pediatric population. In response to the Agency’s written request (original 11/30/2001; amended 4/9/02, 7/3/02, 5/7/04, 6/29/05), the sponsor conducted clinical trials for two indications: schizophrenia (F1D-MC-HGIN) and bipolar disorder (F1D-MC-HGIU) in adolescents, and submitted the study results to the above referenced supplemental NDA on 10/30/2006. -

Regencerx Preferred Medication List/Formulary Regencerx Preferred Effective February 1, 2008 ©2008
RegenceRx Preferred Medication List/Formulary Help to stretch your prescription dollar February1, 2008 ©2008. RegenceRx. All Rights Reserved. ©2008. RegenceRx. Effective February 1,2008 Effective 2 Your Preferred Medication List/Formulary 3 Common Questions and Answers Preferred/Formulary Medications 4-11 • By Category • A to Z Listing 12-17 – Generics 18-22 – Preferred/Formulary Brands Non-Preferred/Non-Formulary Brands 23-27 • Generic and Preferred Brand Alternatives Table of Contents Table Effective February 1, 2008 ©2008. RegenceRx. All Rights Reserved. RegenceRx is dedicated to provide the tools you need to understand your options and be a more informed consumer. This preferred medication list/formulary will help—with the answers you need to access affordable prescription benefits. Determining Cost Newly-introduced How much you pay for a prescription generic medications depends on whether it’s a: When a generic is introduced to the • Generic marketplace, it immediately becomes • Preferred/formulary brand name available at the lowest copay. After a • Non-preferred/non-formulary generic is available, the brand name brand name (brand name medication will only be available at the medication not on the list) non-preferred/non-formulary copay. Your cost is lowest for generic Preferred/formulary medications and highest for non- brand name medications preferred/non-formulary brand name Brand name medications on the medications. You can find specific preferred medication list (PML) coverage and copay information in have been chosen because our your summary of benefits. professional review has shown each Generic medications of them to be high-quality, effective, Generics provide the same and affordable. Preferred/formulary therapeutic benefits of their brand brand name medications are either name counterparts, without the more effective or equally effective—at brand name price. -

Ecstasy and the Concomitant Use of Pharmaceuticals
J.Copeland, P. Dillon & M. Gascoigne Ecstasy and the Concomitant Use of Pharmaceuticals NDARC Technical Report No. 201 ECSTASY AND THE CONCOMITANT USE OF PHARMACEUTICALS Jan Copeland (PhD), Paul Dillon & Michael Gascoigne Technical Report Number 201 ISBN: 1 877027 92 8 ©National Drug and Alcohol Research Centre, University of New South Wales, Sydney, 2004 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. All other rights are reserved. Requests and enquiries concerning reproduction and rights should be addressed to the information manager, National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW 2052, Australia. 2 ACKNOWLEDGEMENT This study and report was funded by the Australian Government Department of Health and Ageing’s National Illicit Drug Strategy. It was conducted concurrently with a study of aspects of information seeking among ecstasy users and there is some replication of the data presented on demographic characteristics and perception of ecstasy related harms in this Technical Report and Technical Report Number 202. 3 TABLE OF CONTENTS ACKNOWLEDGEMENT 3 TABLE OF CONTENTS 4 TABLE OF TABLES 5 EXECUTIVE SUMMARY 6 INTRODUCTION 7 Anti-depressant medication 8 5-hydroxytryptophan 9 Attention Deficit Hyperactivity Disorder 11 Sildenafil Citrate 12 Benzodiazepines 13 Study Ains 14 METHOD 15 Participants 15 Procedure 15 Measures 15 RESULTS 17 Demographics