2016-11-BMF-83 Antidepressants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

Phenibut Overdose in Combination with Fasoracetam: Emerging Drugs of Abuse
Open Access Journal of Clinical Intensive Care and Medicine Case Report Phenibut Overdose in Combination ISSN with Fasoracetam: Emerging Drugs 2639-6653 of Abuse Cristian Merchan1*, Ryan Morgan1, John Papadopoulos1,2 and David Fridman2 1Department of Pharmacy, New York University Langone Medical Center, New York, USA 2New York University School of Medicine, New York, USA *Address for Correspondence: Dr. Cristian INTRODUCTION D Merchan, Pharm.D, BCCCP, Critical Care Pharmacotherapist, Department of Pharmacy, The widespread availability of non-traditional dietary supplements and New York University Langone Medical Center, pharmacologically active substances via the Internet continues to introduce New York, USA, Tel: 516-263-1671; Email: [email protected] mechanisms for inadvertent toxidromes not commonly seen. Consumers are virtually unrestricted in their ability to acquire products purporting augmentation of normal Submitted: 25 October 2016 Approved: 15 December 2016 physiology for the purposes of enhancement, recreation, and/or potential abuse. The Published: 17 December 2016 safety proiles at standard or toxic doses remain largely unknown for many agents that Copyright: 2016 Merchan et al. This is an can be purchased electronically. We report a case of mixed toxicity related to phenibut open access article distributed under the and fosaracetam, both of which are readily available for consumer purchase from Creative Commons Attribution License, which online retailers. Written and verbal consent was obtained for this case presentation. permits unrestricted use, distribution, and reproduction in any medium, provided the CASE REPORT original work is properly cited. Keywords: Nootropic; GABA agonist; Drug of A 27-year-old male with a past medical history of anxiety was discovered abuse; Dietary supplement; Alternative medicine unresponsive on the sidewalk. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Central Valley Toxicology Drug List
Chloroform ~F~ Lithium ~A~ Chlorpheniramine Loratadine Famotidine Acebutolol Chlorpromazine Lorazepam Fenoprofen Acetaminophen Cimetidine Loxapine Fentanyl Acetone Citalopram LSD (Lysergide) Fexofenadine 6-mono- Clomipramine acetylmorphine Flecainide ~M~ Clonazepam a-Hydroxyalprazolam Fluconazole Maprotiline Clonidine a-Hydroxytriazolam Flunitrazepam MDA Clorazepate Albuterol Fluoxetine MDMA Clozapine Alprazolam Fluphenazine Medazepam Cocaethylene Amantadine Flurazepam Meperidine Cocaine 7-Aminoflunitrazepam Fluvoxamine Mephobarbital Codeine Amiodarone Fosinopril Meprobamate Conine Amitriptyline Furosemide Mesoridazine Cotinine Amlodipine Methadone Cyanide ~G~ Amobarbital Methanol Cyclobenzaprine Gabapentin Amoxapine d-Methamphetamine Cyclosporine GHB d-Amphetamine l-Methamphetamine Glutethamide l-Amphetamine ~D~ Methapyrilene Guaifenesin Aprobarbital Demoxepam Methaqualone Atenolol Desalkylfurazepam ~H~ Methocarbamol Atropine Desipramine Halazepam Methylphenidate ~B~ Desmethyldoxepin Haloperidol Methyprylon Dextromethoraphan Heroin Metoclopramide Baclofen Diazepam Hexobarbital Metoprolol Barbital Digoxin Hydrocodone Mexiletine Benzoylecgonine Dihydrocodein Hydromorphone Midazolam Benzphetamine Dihydrokevain Hydroxychloroquine Mirtazapine Benztropine Diltiazem Hydroxyzine Morphine (Total/Free) Brodificoum Dimenhydrinate Bromazepam ~N~ Diphenhydramine ~I~ Bupivacaine Nafcillin Disopyramide Ibuprofen Buprenorphine Naloxone Doxapram Imipramine Bupropion Naltrexone Doxazosin Indomethacin Buspirone NAPA Doxepin Isoniazid Butabarbital Naproxen -
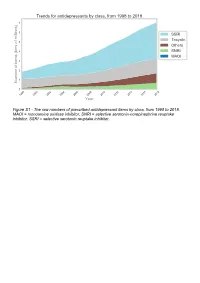
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Depot Antipsychotic Injections
Mental Health Services Good Practice Statement For The Use Of DEPOT & LONG ACTING ANTIPSYCHOTIC INJECTIONS Date of Revision: February 2020 Approved by: Mental Health Prescribing Management Group Review Date: CONSULTATION The document was sent to Community Mental Health teams, Pharmacy and the Psychiatric Advisory Committee for comments prior to completion. KEY DOCUMENTS When reading this policy please consult the NHS Consent Policy when considering any aspect of consent National Institute for Clinical Excellence CG178 (2014), Psychosis and schizophrenia in adults: prevention and management NHS Lothian (2011), Depot Antipsychotic Guidance, Maudsley (2015), Prescribing Guidelines in Psychiatry 12th edition. UKPPG (2009) Guidance on the Administration to Adults of Oil based Depot and other Long Acting Intramuscular Antipsychotic Injections Summary of Product Characteristics for: Clopixol (Zuclopethixol Decanoate) Flupenthixol Decanoate Haldol (Haloperidol Decanoate) Risperdal Consta Xeplion (Paliperidone Palmitate) ZypAdhera (Olanzapine Embonate) Abilify Maintena (Aripiprazole) 1 CONTENTS REFERENCES/KEY DOCUMENTS …………………………………………………………… 1 INTRODUCTION …………………………………………………………………………………. 3 SCOPE …………………………………………………………………………………………….. 3 DEFINITIONS ……………………………………………………………………………………... 3 CHOICE OF DRUG AND DOSAGE SELECTION ……………………………………………. 3 SIDE EFFECTS ……………………………………………………………….………………….. 5 PRESCRIBING …………………………………………………………………………………… 5 ADMINISTRATION ………………………………………………………………………………. 7 RECORDING ADMINISTRATION ……………………………………………………………… 7 -

Deanxit SPC.Pdf
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deanxit film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Flupentixol 0.5 mg (as 0.584 mg flupentixol dihydrochloride) Melitracen 10 mg (as 11.25 mg melitracen hydrochloride) Excipients with known effect: Lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablets. Round, biconvex, cyclamen, film-coated tablets. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Anxiety Depression Asthenia. Neurasthenia. Psychogenic depression. Depressive neuroses. Masked depression. Psychosomatic affections accompanied by anxiety and apathy. Menopausal depressions. Dysphoria and depression in alcoholics and drug-addicts. 4.2 Posology and method of administration Posology Adults Usually 2 tablets daily: morning and noon. In severe cases the morning dose may be increased to 2 tablets. The maximum dose is 4 tablets daily. Older people (> 65 years) 1 tablet in the morning. In severe cases 1 tablet in the morning and 1 at noon. Maintenance dose: Usually 1 tablet in the morning. SPC Portrait-REG_00051968 20v038 Page 1 of 10 In cases of insomnia or severe restlessness additional treatment with a sedative in the acute phase is recommended. Paediatric population Children and adolescents (<18 years) Deanxit is not recommended for use in children and adolescents due to lack of data on safety and efficacy. Reduced renal function Deanxit can be given in the recommended doses. Reduced liver function Deanxit can be given in the recommended doses. Method of administration The tablets are swallowed with water. 4.3 Contraindications Hypersensitivity to flupentixol and melitracen or to any of the excipients listed in section 6.1. -

Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs
ISSN 2380-5064 | The Arsenal is published by the Augusta University Libraries | http://guides.augusta.edu/arsenal Volume 4, Issue 1 (2021) Special Edition Issue CONJUGATION OF TRYPTAMINE TO BIOLOGICALLY ACTIVE CARBOXYLIC ACIDS IN ORDER TO CREATE PRODRUGS Colin Miller, Jr. and Iryna O. Lebedyeva Citation Miller, C., Jr., & Lebedyeva, I. O. (2021). Conjugation of tryptamine to biologically active carboxylic acids in order to create prodrugs. The Arsenal: The Undergraduate Research Journal of Augusta University, 4(1), 26. http://doi.org/10.21633/issn.2380.5064/s.2021.04.01.26 © Miller, Jr. and Lebedyeva 2021. This open access article is distributed under a Creative Commons Attribution NonCommercial-NoDerivs 2.0 Generic License (https://creativecommons.org/licenses/by-nc-nd/2.0/). Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs Presenter(s): Colin Miller, Jr. Author(s): Colin Miller Jr. and Iryna O. Lebedyeva Faculty Sponsor(s): Iryna O. Lebedyeva, PhD Affiliation(s): Department of Chemistry and Physics (Augusta Univ.) ABSTRACT A number of blood and brain barrier penetrating neurotransmitters contain polar functional groups. Gamma-aminobutyric acid (GABA) is an amino acid, which is one of the primary inhibitory neurotransmitter in the brain and a major inhibitory neurotransmitter in the spinal cord. Antiepileptic medications such as Gabapentin, Phenibut, and Pregabalin have been developed to structurally represent GABA. These drugs are usually prescribed for the treatment of neuropathic pain. Since these drugs contain a polar carboxylic acid group, it affects their ability to penetrate the blood and brain barrier. To address the low bioavailability and tendency for intramolecular cyclization of Gabapentin, its less polar prodrug Gabapentin Enacarbil has been approved in 2011. -

Comprehensive Panel, Blood
COMPREHENSIVE PANEL, BLOOD Blood Specimens (Order Code 70510) Alcohols Analgesics, cont. Anticonvulsants, cont. Antihistamines Acetone Norbuprenorphine Gabapentin Brompheniramine Ethanol Nortramadol Lamotrigine Cetirizine Isopropanol Oxaprozin Levetiracetam Chlorpheniramine Methanol Pentoxifylline Methsuximide Cyclizine Amphetamines Phenacetin Phenytoin Diphenhydramine Amphetamine Phenylbutazone Pregabalin Doxylamine BDB Piroxicam Primidone Fexofenadine Benzphetamine Salicylic Acid* Topiramate Guaifenesin Ephedrine Sulindac* Zonisamide Hydroxyzine MDA Tapentadol Antidepressants Loratadine MDMA Tizanidine Amitriptyline Oxymetazoline* Mescaline* Tolmetin Amoxapine Pyrilamine Methcathinone Tramadol Bupropion Tetrahydrozoline Methamphetamine Anesthetics Citalopram Triprolidine Phentermine Benzocaine Clomipramine Antipsychotics PMA Bupivacaine Desipramine 9-hydroxyrisperidone Phenylpropanolamine Etomidate Desmethylclomipramine Aripiprazole Pseudoephedrine Ketamine Dosulepin Buspirone Analgesics Lidocaine Doxepin Chlorpromazine Acetaminophen Mepivacaine Duloxetine Clozapine Baclofen Methoxetamine Fluoxetine Fluphenazine Buprenorphine Midazolam Fluvoxamine Haloperidol Carisoprodol Norketamine Imipramine Mesoridazine Cyclobenzaprine Pramoxine* 1,3-chlorophenylpiperazine (mCPP) Norclozapine Diclofenac Procaine Mianserin* Olanzapine Etodolac Rocuronium Mirtazapine Perphenazine Fenoprofen Ropivacaine Nefazodone Pimozide Hydroxychloroquine Antibiotics Norclomipramine Prochlorperazine Ibuprofen Azithromycin* Nordoxepin Quetiapine Ketoprofen Chloramphenicol* -

Toxicology Report Division of Toxicology Daniel D
Franklin County Forensic Science Center Office of the Coroner Anahi M. Ortiz, M.D. 2090 Frank Road Columbus, Ohio 43223 Toxicology Report Division of Toxicology Daniel D. Baker, Chief Toxicologist Casey Goodson Case # LAB-20-5315 Date report completed: January 28, 2021 A systematic toxicological analysis has been performed and the following agents were detected. Postmortem Blood: Gray Top Thoracic ELISA Screen Acetaminophen Not Detected ELISA Screen Barbiturates Not Detected ELISA Screen Benzodiazepines Not Detected ELISA Screen Benzoylecgonine Not Detected ELISA Screen Buprenorphine Not Detected ELISA Screen Cannabinoids See Confirmation ELISA Screen Fentanyl Not Detected ELISA Screen Methamphetamine Not Detected ELISA Screen Naltrexone/Naloxone Not Detected ELISA Screen Opiates Not Detected ELISA Screen Oxycodone/Oxymorphone Not Detected ELISA Screen Salicylates Not Detected ELISA Screen Tricyclics Not Detected Page 1 of 4 Casey Goodson Case # LAB-20-5315 GC/FID Ethanol Not Detected GC/MS Acidic/Neutral Drugs None Detected GC/MS Nicotine Positive GC/MS Cotinine Positive Reference Lab Delta-9-THC 13 ng/mL Reference Lab 11-Hydroxy-Delta-9-THC 1.2 ng/mL Reference Lab 11-Nor-9-Carboxy-Delta-9-THC 15 ng/mL Postmortem Urine: Gray Top Urine GC/MS Cotinine Positive This report has been verified as accurate and complete by ______________________________________ Daniel D. Baker, M.S., F-ABFT Cannabinoid quantitations in blood were performed by NMS Labs, Horsham, PA. Page 2 of 4 Casey Goodson Case # LAB-20-5315 Postmortem Toxicology Scope of Analysis Franklin County Coroner’s Office Division of Toxicology Enzyme Linked Immunosorbant Assay (ELISA) Blood Screen: Qualitative Presumptive Compounds/Classes: Acetaminophen (cut-off 10 µg/mL), Benzodiazepines (cut-off 20 ng/mL), Benzoylecgonine (cut-off 50 ng/mL), Cannabinoids (cut-off 40 ng/mL), Fentanyl (cut-off 1 ng/mL), Methamphetamine/MDMA (cut-off 50 ng/mL), Opiates (cut-off 40 ng/mL), Oxycodone/Oxymorphone (cut-off 40 ng/mL), Salicylates (50 µg/mL). -

Determination of Antidepressants in Human Plasma by Modified Cloud
pharmaceuticals Article Determination of Antidepressants in Human Plasma by Modified Cloud-Point Extraction Coupled with Mass Spectrometry El˙zbietaGniazdowska 1,2 , Natalia Korytowska 3 , Grzegorz Kłudka 3 and Joanna Giebułtowicz 3,* 1 Łukasiewicz Research Network, Industrial Chemistry Institute, 8 Rydygiera, 01-793 Warsaw, Poland; [email protected] 2 Department of Bioanalysis and Drugs Analysis, Doctoral School, Medical University of Warsaw, 61 Zwirki˙ i Wigury, 02-091 Warsaw, Poland 3 Department of Bioanalysis and Drugs Analysis, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha, 02-097 Warsaw, Poland; [email protected] (N.K.); [email protected] (G.K.) * Correspondence: [email protected] Received: 5 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Cloud-point extraction (CPE) is rarely combined with liquid chromatography coupled to mass spectrometry (LC–MS) in drug determination due to the matrix effect (ME). However, we have recently shown that ME is not a limiting factor in CPE. Low extraction efficiency may be improved by salt addition, but none of the salts used in CPE are suitable for LC–MS. It is the first time that the influences of a volatile salt—ammonium acetate (AA)—on the CPE extraction efficiency and ME have been studied. Our modification of CPE included also the use of ethanol instead of acetonitrile to reduce the sample viscosity and make the method more environmentally friendly. We developed and validated CPE–LC–MS for the simultaneous determination of 21 antidepressants in plasma that can be useful for clinical and forensic toxicology. The selected parameters included Triton X-114 concentration (1.5 and 6%, w/v), concentration of AA (0, 10, 20 and 30%, w/v), and pH (3.5, 6.8 and 10.2). -

ANTIDEPRESSANTS in PLASMA by LC/MS Code LC84010
ANTIDEPRESSANTS IN PLASMA BY LC/MS Code LC84010 This product fulfills all the requirements of Directive 98/79/ EC on in vitro diagnostic medical devices (IVD). The declaration of conformity (CE) is available upon request. Release N° 001 Antidepressants in plasma by LC/MS January 2020 CLINICAL PHARMACOLOGY INTRODUCTION Antidepressants are a class of medications used in emotional and mood disorders, which can evolve into psychosis and other symptoms such as anhedonia, self-devaluation, indecision, lack of appetite, hyperphagia, insomnia, hypersomnia, recurring thoughts of suicide or murder and somatization of pain. These drugs are divided into various classes according to their mechanism of action: - MAOIs (monoamine oxidase inhibitors); - TCA (tricyclic antidepressants); - SSRIs (selective serotonin reuptake inhibitors); - SNRI (serotonin and norepinephrine reuptake inhibitors); - NARI (noradrenaline reuptake inhibitors); - NDRI (dopamine and noradrenaline reuptake inhibitors); - DRI (dopamine reuptake inhibitors); - SSRE (serotonergic drugs); - NASSA (specific noradrenergic and serotonergic antidepressants); - Melatonergic drugs. Given the large number of antidepressants on the market and their increasing use, there is a need for physicians to monitor their plasma concentration in order to avoid episodes of overdose. This method allows the determination of a number of antidepressant drugs and their main active metabolites: amitriptyline, nortriptyline, clomipramine, norclomipramine, desipramine, desvenlafaxine, doxepine, nordoxepine, duloxetine,