Deanxit SPC.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -
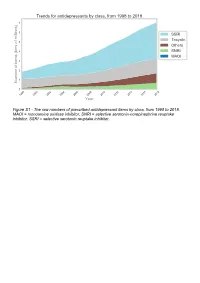
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Depot Antipsychotic Injections
Mental Health Services Good Practice Statement For The Use Of DEPOT & LONG ACTING ANTIPSYCHOTIC INJECTIONS Date of Revision: February 2020 Approved by: Mental Health Prescribing Management Group Review Date: CONSULTATION The document was sent to Community Mental Health teams, Pharmacy and the Psychiatric Advisory Committee for comments prior to completion. KEY DOCUMENTS When reading this policy please consult the NHS Consent Policy when considering any aspect of consent National Institute for Clinical Excellence CG178 (2014), Psychosis and schizophrenia in adults: prevention and management NHS Lothian (2011), Depot Antipsychotic Guidance, Maudsley (2015), Prescribing Guidelines in Psychiatry 12th edition. UKPPG (2009) Guidance on the Administration to Adults of Oil based Depot and other Long Acting Intramuscular Antipsychotic Injections Summary of Product Characteristics for: Clopixol (Zuclopethixol Decanoate) Flupenthixol Decanoate Haldol (Haloperidol Decanoate) Risperdal Consta Xeplion (Paliperidone Palmitate) ZypAdhera (Olanzapine Embonate) Abilify Maintena (Aripiprazole) 1 CONTENTS REFERENCES/KEY DOCUMENTS …………………………………………………………… 1 INTRODUCTION …………………………………………………………………………………. 3 SCOPE …………………………………………………………………………………………….. 3 DEFINITIONS ……………………………………………………………………………………... 3 CHOICE OF DRUG AND DOSAGE SELECTION ……………………………………………. 3 SIDE EFFECTS ……………………………………………………………….………………….. 5 PRESCRIBING …………………………………………………………………………………… 5 ADMINISTRATION ………………………………………………………………………………. 7 RECORDING ADMINISTRATION ……………………………………………………………… 7 -

Trazodone Is Not As Effective As Other Antidepressants in Treating a Patient with Major Depressive Disorder
Case Report Taiwanese Journal of Psychiatry (Taipei) Vol. 26 No. 4 2012 • 311 • Trazodone Is Not as Effective as Other Antidepressants in Treating a Patient with Major Depressive Disorder Kah Kheng Goh, M.D.1, Weng-Kin Tam, M.D.1, Winston W. Shen, M.D.1, 2* Background: Trazodone in clinical use is ineffective because of its sed ation to let patient receive an adequate dose for treating a patient with major depressive disorder. We are report a case report to demonstrate this clinical effi cacy issue in the treatment with trazodone. Case Report: A 83-year old male Taiwanese patient had suffered from MDD for six months. He did not response to the treatment of trazodone 150 mg/day for four months, but consequently, he responded to the treatment of milnacipran 100 mg/day or mirtazapine 30 mg/day. Conclusion: Psy- chiatrists in Taiwan should be alert to the fact that trazodone is not as effective as other antidepressants, and that trazodone should not be prescribed as a single anti- depressant in treating an MDD patient. Key words: antidepressant therapy, suicide, venlafaxine, bupropion (Taiwanese Journal of Psychiatry [Taipei] 2012; 26: 311-5) guideline in the paragraph in describing individu- Introduction al drug trazodone also indicates “other investiga- tors found trazodone to be less effective than other The practice guideline of the American antidepressant medications” [1]. In this case re- Psychiatric Association in the paragraph of intro- port here, we are reporting a case of a major de- ducing antidepressants states “Although some pressive disorder (MDD) patient, who did not re- studies have suggested superiority of one mecha- spond to daily dose of 150 mg of trazodone, but nism of action over another, there are no replica- responded well to antidepressants 100 mg of mil- ble or robust fi ndings to establish a clinically nacipran per day and maybe 30 mg of mirtazapine meaningful difference. -

020592Orig1s040s041
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 020592Orig1s040s041 MEDICAL REVIEW(S) M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: July 18, 2008 FROM: Ni A. Khin, M.D. Team Leader Division of Psychiatry Products, HFD-130 TO: NDA 20-592/SE5-040 (bipolar I disorder, acute mania) NDA 20-592/SE5-041 (schizophrenia) (This overview should be filed with the 02-05-2008 submission in response to the Agency’s Approvable Letter dated 04-30-2007) SUBJECT: Recommendation of an approvable action for use of Zyprexa (olanzapine) in the treatment of 1) Bipolar I disorder, Mania, and 2) schizophrenia in Adolescents. 1. BACKGROUND Zyprexa (olanzapine) is an atypical antipsychotic agent, approved in the U.S. for treatment of schizophrenia and bipolar disorder, mania or mixed episodes, as monotherapy (both acute and maintenance) or combination therapy in adults. It is available as oral 2.5, 5, 10, 15, or 20 mg strength tablets; 5, 10, 15, or 20 mg oral disintegrating tablets (Zydis). The target dose for adults with schizophrenia is 10 mg/day. Zyprexa intramuscular injection (10 mg) is indicated for agitation associated with schizophrenia and Bipolar I Mania. Currently, two atypical antipsychotic drugs, Risperdal and Abilify, are approved for treatment of schizophrenia and bipolar disorder in the pediatric population. In response to the Agency’s written request (original 11/30/2001; amended 4/9/02, 7/3/02, 5/7/04, 6/29/05), the sponsor conducted clinical trials for two indications: schizophrenia (F1D-MC-HGIN) and bipolar disorder (F1D-MC-HGIU) in adolescents, and submitted the study results to the above referenced supplemental NDA on 10/30/2006. -

Thioxanthene Derivates As Sole Anti-Infective Agents
(19) & (11) EP 2 114 406 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/496 (2006.01) A61P 31/04 (2006.01) 25.05.2011 Bulletin 2011/21 A61P 33/00 (2006.01) (21) Application number: 08700129.3 (86) International application number: PCT/DK2008/000005 (22) Date of filing: 07.01.2008 (87) International publication number: WO 2008/080408 (10.07.2008 Gazette 2008/28) (54) THIOXANTHENE DERIVATESAS SOLEANTI- INFECTIVEAGENTS FORUSE INT HE TREATMENT OF INFECTIOUS DISEASES THIOXANTHEN-DERIVATE ALS ALLEINVERTRETER DER ANTIINFEKTIVA ZUR VERWENDUNG IN DER BEHANDLUNG VON INFEKTIONSKRANKHEITEN DÉRIVÉS DE THIOXANTHÈNE COMME SEULS AGENTS ANTIINFECTIEUX POUR LEUR UTILISATION DANS LE TRAITEMENT DES MALADIES INFECTIEUSES (84) Designated Contracting States: • MORTENSEN I ET AL: "THE ANTIBACTERIAL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR ACTIVITY OF THE HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT PSYCHOPHARMACOLOGICAL AGENT RO SE SI SK TR CLOPENTHIXOL AND ITS TWO MAIN METABOLITES" ACTA PATHOLOGICA (30) Priority: 05.01.2007 PCT/DK2007/000006 MICROBIOLOGICASCANDINAVICA, SECTION B, XX, XX, vol. 95B, no. 6, 1987, pages 355-359, (43) Date of publication of application: XP008066133 ISSN: 0108-0180 11.11.2009 Bulletin 2009/46 • KOCISKO ET AL: "Comparison of protease- resistant prion protein inhibitors in cell cultures (60) Divisional application: infected with two strains of mouse and sheep 10181816.9 / 2 301 545 scrapie" NEUROSCIENCE LETTERS, LIMERICK, IE, vol. 388, no. 2, 11 November 2005 (2005-11-11), (73) Proprietor: BKG Pharma ApS pages 106-111, XP005042190 ISSN: 0304-3940 2920 Charlottenlund (DK) • KAISER C ET AL: "Analogs of phenothiazines. -

Flupentixol 0.5 Mg & Melitracen 10 Mg Tablet
Flupentixol 0.5 mg & Melitracen 10 mg Tablet COMPOSITION Each film coated tablet contains Flupentixol hydrochloride BP equivalent to 0.5 mg Flupentixol and Melitracen hydrochloride INN equivalent to 10 mg Melitracen. PHARMACOKINETICS Maximum serum concentration is reached in about 4 hours after oral administration of Flupentixol and Melitracen. The half-life of Flupentixol is about 35 hours and that of Melitracen is about 19 hours. The combination of Flupentixol and Melitracen does not seem to influence the pharmacokinetic properties of the individual compounds. INDICATION Psychogenic depression, Depressive neurosis, Psychosomatic affections accompanied by anxiety and apathy, menopausal depression, depression in alcoholics and drug-addicts. DOSAGE AND ADMINISTRATION Adults : Usually 2 tablets daily, in morning and afternoon. In severe cases, the morning dose may be increased to 2 tablets. Elderly patients : 1 tablet in the morning. Maintenance dose : Usually 1 tablet in the morning or as directed by the physician. CONTRAINDICATION It is contraindicated in the immediate recovery phase after myocardial infarction, defects in bundle-branch conduction, untreated narrow angle glaucoma and in acute alcohol, barbiturate & opioid intoxications. Not recommended for excitable patients since its activating effect may lead to exaggeration of these characteristics. SIDE EFFECT In the recommended doses, side effects are rare. These could be transient restlessness and insomnia. PRECAUTION If previously the patient has been treated with tranquilizers with sedative effect these should be withdrawn gradually. DRUG INTERACTION This preparation may enhance the response to alcohol, barbiturates and other CNS depressants. Simultaneous administrantion of MAO-inhibitors may cause hypertensive crisis. USE IN PREGNANCY AND LACTATION The safety of this drug has not been established in pregnancy and lactation. -

Depression and Structural Factors Are Associated with Symptoms in Patients of Irritable Bowel Syndrome with Diarrhea
J Neurogastroenterol Motil, Vol. 26 No. 4 October, 2020 pISSN: 2093-0879 eISSN: 2093-0887 https://doi.org/10.5056/jnm19166 JNM Journal of Neurogastroenterology and Motility Original Article Depression and Structural Factors Are Associated With Symptoms in Patients of Irritable Bowel Syndrome With Diarrhea Jia Lu,1 Lili Shi,2 Dan Huang,3 Wenjuan Fan,1 Xiaoqing Li,1 Liming Zhu,1 Jing Wei,2 and Xiucai Fang1* Departments of 1Gastroenterology and 2Psychological Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; and 3Department of Gastroenterology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China Background/Aims A strong correlation between depression and irritable bowel syndrome with diarrhea (IBS-D) has been identified. The aim of this study is to identify the correlations among depression, structural factors, gastrointestinal (GI) and extra-GI symptoms, and efficacy of neuromodulators in patients with IBS-D. Methods Patients meeting the Rome III Diagnostic Criteria for IBS-D were enrolled. The intestinal symptoms and psychological states were evaluated using IBS-specific symptom questionnaires and Hamilton Depression Rating Scale. Results In total, 410 patients with IBS-D were enrolled, 28.8% (118/410) had comorbid depression. Patients with depression did not readily experience improvement in abdominal pain/discomfort after defecation, and had a higher prevalence of passing mucus, overlapping functional dyspepsia, and extra-GI symptoms. The structural factor “mental disorders” significantly correlated with main bowel symptom score and degree of pre-defecation abdominal pain/discomfort. No structural factor significantly correlated with bowel movements or stool form. -

Quinolizidine-Derived Lucanthone and Amitriptyline Analogues Endowed with Potent Antileishmanial Activity
pharmaceuticals Article Quinolizidine-Derived Lucanthone and Amitriptyline Analogues Endowed with Potent Antileishmanial Activity Michele Tonelli 1,* , Anna Sparatore 2,* , Nicoletta Basilico 3,* , Loredana Cavicchini 3, Silvia Parapini 4 , Bruno Tasso 1 , Erik Laurini 5 , Sabrina Pricl 5,6 , Vito Boido 1 and Fabio Sparatore 1 1 Dipartimento di Farmacia, Università degli Studi di Genova, Viale Benedetto XV, 3, 16132 Genova, Italy; [email protected] (B.T.); [email protected] (V.B.); [email protected] (F.S.) 2 Dipartimento di Scienze Farmaceutiche, Università degli Studi di Milano, Via Mangiagalli 25, 20133 Milano, Italy 3 Dipartimento di Scienze Biomediche Chirurgiche e Odontoiatriche, Università degli Studi di Milano, Via Pascal 36, 20133 Milano, Italy; [email protected] 4 Dipartimento di Scienze Biomediche per la Salute, Università degli Studi di Milano, Via Mangiagalli 31, 20133 Milano, Italy; [email protected] 5 Molecular Biology and Nanotechnology Laboratory (MolBNL@UniTS), DEA, Piazzale Europa 1, 34127 Trieste, Italy; [email protected] (E.L.); [email protected] (S.P.) 6 Department of General Biophysics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland * Correspondence: [email protected] (M.T.); [email protected] (A.S.); [email protected] (N.B.) Received: 4 October 2020; Accepted: 21 October 2020; Published: 25 October 2020 Abstract: Leishmaniases are neglected diseases that are endemic in many tropical and sub-tropical Countries. Therapy is based on different classes of drugs which are burdened by severe side effects, occurrence of resistance and high costs, thereby creating the need for more efficacious, safer and inexpensive drugs. -

N-Alkylamino Derivates of Aromatic, Tricyclic Compounds in the Treatment of Drug-Resistant Protozoal Infections
Europaisches Patentamt 0 338 532 European Patent Office Qv Publication number: A2 Office europeen des brevets EUROPEAN PATENT APPLICATION 31/54 © Application number: 89107027.8 © mt.ci.4.A61K 31/38 , A61K @ Date of filing: 19.04.89 © Priority: 20.04.88 US 183858 © Applicant: MERRELL DOW 12.09.88 US 243524 PHARMACEUTICALS INC. 2110 East Galbraith Road @. Date of publication of application: Cincinnati Ohio 45215-6300(US) 25.10.89 Bulletin 89/43 © Inventor: Bitonti, Alan J. © Designated Contracting States: 7854 Carraway Court AT BE CH DE ES FR GB GR IT LI LU NL SE Maineville Ohio 45039(US) Inventor: McCann, Peter P. 3782 Cooper Road Cincinnati Ohio 45241 (US) Inventor: Sjoerdsma, Albert 5475 Waring Drive Cincinnati Ohio 45243(US) © Representative: Vossius & Partner Siebertstrasse 4 P.O. Box 86 07 67 D-8000 Munchen 86(DE) © N-alkylamino derivates of aromatic, tricyclic compounds in the treatment of drug-resistant protozoal infections. © Drug-resistant protozoal infection, particularly, drug-resistant malarial infection in humans, can be effectively treated with standard antiprotozoal agents if administered in conjunction with a tricyclic N-aminoalkyi iminodiben- zyl, dibenzylcycloheptane and cycloheptene, phenothiazine, dibenzoxazepine, dibenzoxepine, or thioxanthene derivative. CM < CM CO in oo CO CO LU Xerox Copy Centre EP 0 338 532 A2 N-ALKYLAMINO DERIVATIVES OF AROMATIC, TRICYCLIC COMPOUNDS IN THE TREATMENT OF DRUG- RESISTANT PROTOZOAL INFECTIONS This invention relates to the use of certain N-(aminoalkyl) derivatives of iminodibenzyl, dibenzyl- cycioheptane and cycloheptene, phenothiazine, dibenzoxazepine, dibenzoxepine, and thioxanthene in the treatment of drug-resistant malaria and in the treatment of other drug-resistant protozoal infections.