Product Monograph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aristada™ (Aripiprazole Lauroxil)
Aristada™ (aripiprazole lauroxil) – New Drug Approval • On October 5, 2015, Alkermes’ announced the FDA approval of Aristada (aripiprazole lauroxil) extended-release injection, an atypical antipsychotic, for the treatment of schizophrenia. • Schizophrenia is a chronic, severe and disabling brain disorder affecting an estimated 2.4 million Americans. Typically, symptoms include hearing voices, believing other people are reading their minds or controlling their thoughts, and being suspicious or withdrawn. • Aristada’s approval was based on data from a double-blind, placebo-controlled 12-week trial involving 622 patients with schizophrenia. In addition, the efficacy of Aristada was established, in part, on the basis of efficacy data from trials with oral aripiprazole. — Aristada significantly improved symptoms of schizophrenia compared to placebo at day 85. • Similar to other atypical antipsychotics, Aristada carries a boxed warning for increased mortality in elderly patients with dementia-related psychosis. • Other warnings and precautions for Aristada include cerebrovascular adverse reactions, including stroke; neuroleptic malignant syndrome; tardive dyskinesia; metabolic changes; orthostatic hypotension; leukopenia, neutropenia, and agranulocytosis; seizures; potential for cognitive and motor impairment; body temperature regulation; and dysphagia. • The most common adverse reaction (≥ 5% and at least twice that for placebo) with Aristada use was akathisia. • Aristada is administered by intramuscular injection in the deltoid (441 mg dose only) or gluteal (441 mg, 662 mg, or 882 mg) muscle by a healthcare professional. — Aristada can be initiated at a monthly dose (441 mg, 662 mg or 882 mg) or every 6 week dose (882 mg). — For patients naïve to aripiprazole, tolerability should be established with oral aripiprazole prior to initiating treatment with Aristada. -

Lipid-Based Depots: Manufacturing, Administration and Interactions of Protein Drugs with Lipid Formulations
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Lipid-based depots: manufacturing, administration and interactions of protein drugs with lipid formulations Michaela Maria Breitsamer aus Starnberg, Deutschland 2019 II ERKLÄRUNG Diese Dissertation wurde im Sinne von § 7 der Promotionsordnung vom 28. November 2011 von Herrn Prof. Dr. Gerhard Winter betreut. EIDESSTATTLICHE VERSICHERUNG Diese Dissertation wurde eigenständig und ohne unerlaubte Hilfe erarbeitet. Wolfratshausen, den 20.05.2019 ________________________________ (Michaela Breitsamer) Dissertation eingereicht am: 20.05.2019 1. Gutachter: Prof. Dr. Gerhard Winter 2. Gutachter: Prof. Dr. Wolfgang Frieß Mündliche Prüfung am: 25.06.2019 III IV FOR MY FAMILY “The way to get started is to quit talking and begin doing” Walt Disney (1901 – 1966) V VI ACKNOWLEDGEMENTS The present thesis was prepared between March 2015 and December 2018 at the Department of Pharmacy, Pharmaceutical Technology and Biopharmaceutics at the Ludwig-Maximilians Universität (LMU) in Munich under the supervision of Prof. Dr. Gerhard Winter. First of all, I would like to express my deepest gratitude to my supervisor Prof. Dr. Gerhard Winter for giving me the opportunity to join his research group and to work on this extremely interesting and interdisciplinary project. I really appreciated his scientific input throughout all phases of this work, his elaborate advice and his guidance, which also contributed to my personal development over the last years. Furthermore, I would like to thank him for the outstanding working and team atmosphere, which he created at the chair, for supporting my participation in scientific conferences and for initiating and motivating me to the numerous collaborations which contributed to a successful thesis. -

Food and Drugs Regulations
LAWS OF TRINIDAD AND TOBAGO 22 Chap. 30:01 Food and Drugs SUBSIDIARY LEGISLATION FOOD AND DRUGS REGULATIONS ARRANGEMENT OF REGULATIONS REGULATION 1. Citation. 2. Requirements prescribed by regulation. 3. Interpretation. 4. Request to Director. 5. Functions, duties, responsibilities of Inspectors. 6. Certificate of appointment. 7. Taking of photographs. 8. Taking samples and detention pending further examination. 9. Violation of Act or Regulations and relabelling or recondition ing of food, drug, cosmetic or device. 10. Issue of certificate. 11. Taking a sample, notification of intention and division of sample. 12. Division an interference and objection to procedure by owner or person. 13. Certificate of Analysis. 14. Definition of terms in Part II. 15. Offence to sell unlabelled food. 16. Labelling of package. 17. Declaration of net contents not required on certain labels. 18. List of ingredients not required on certain labels. 19. Declaration not required. 20. Declaration not required to indicate presence of flavouring. 21. Dried or dehydrated products. 22. Food from vending machine. 23. Non application of regulation 16. 24. Standard for a food. 25. Name of designation given to standard, grade or definition. 26. Adulteration of food. 27. Non-adulteration. 28. Contents of package. 29. Display of information on label. 30. First Schedule. 31. Offence. 32. Definition of terms in Part III. 33. Labelling of drug. 34. Contents of label. 35. Label on bulk package. 36. Drug sold on prescription. 37. Packing cases. LAWS OF TRINIDAD AND TOBAGO Food and Drugs Chap. 30:01 23 Food and Drugs Regulations [Subsidiary} REGULATION 38. Name and proportion of drug to be stated on label. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Pharmacotherapy, Drug-Drug Interactions and Potentially
medRxiv preprint doi: https://doi.org/10.1101/2021.03.31.21254518; this version posted April 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Pharmacotherapy, drug-drug interactions and potentially inappropriate medication in depressive disorders Jan Wolff1,2,3, Pamela Reißner4, Gudrun Hefner5, Claus Normann2, Klaus Kaier6, Harald Binder6, Christoph Hiemke7, Sermin Toto8, Katharina Domschke2, Michael Marschollek1, Ansgar Klimke4,9 1 Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Germany. 2 Department of Psychiatry and Psychotherapy, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany. 3 Evangelical Foundation NeuerKerode, Germany. 4 Vitos Hochtaunus, Friedrichsdorf, Germany. 5 Vitos Clinic for Forensic Psychiatry, Eltville, Germany 6 Institute of Medical Biometry and Statistics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany. 7 Department of Psychiatry and Psychotherapy, University Medical Center Mainz, Germany. 8 Department of Psychiatry, Social Psychiatry and Psychotherapy, Hannover Medical School, Germany. 9 Heinrich-Heine-University Düsseldorf, Germany. ___ Correspondence Dr. Jan Wolff, Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Hannover, Germany. Address: Karl- Wiechert-Allee 3, 30625 Hannover. Email: [email protected], wolff.jan@mh- hannover.de, ORCID: https://orcid.org/0000-0003-2750-0606 Key words (MeSH) Depression, Polypharmacy, Antidepressants, Hospitals, Drug Interactions, Psychiatry NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Appendix 13C: Clinical Evidence Study Characteristics Tables
APPENDIX 13C: CLINICAL EVIDENCE STUDY CHARACTERISTICS TABLES: PHARMACOLOGICAL INTERVENTIONS Abbreviations ............................................................................................................ 3 APPENDIX 13C (I): INCLUDED STUDIES FOR INITIAL TREATMENT WITH ANTIPSYCHOTIC MEDICATION .................................. 4 ARANGO2009 .................................................................................................................................. 4 BERGER2008 .................................................................................................................................... 6 LIEBERMAN2003 ............................................................................................................................ 8 MCEVOY2007 ................................................................................................................................ 10 ROBINSON2006 ............................................................................................................................. 12 SCHOOLER2005 ............................................................................................................................ 14 SIKICH2008 .................................................................................................................................... 16 SWADI2010..................................................................................................................................... 19 VANBRUGGEN2003 .................................................................................................................... -
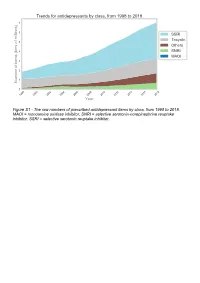
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Depot Antipsychotic Injections
Mental Health Services Good Practice Statement For The Use Of DEPOT & LONG ACTING ANTIPSYCHOTIC INJECTIONS Date of Revision: February 2020 Approved by: Mental Health Prescribing Management Group Review Date: CONSULTATION The document was sent to Community Mental Health teams, Pharmacy and the Psychiatric Advisory Committee for comments prior to completion. KEY DOCUMENTS When reading this policy please consult the NHS Consent Policy when considering any aspect of consent National Institute for Clinical Excellence CG178 (2014), Psychosis and schizophrenia in adults: prevention and management NHS Lothian (2011), Depot Antipsychotic Guidance, Maudsley (2015), Prescribing Guidelines in Psychiatry 12th edition. UKPPG (2009) Guidance on the Administration to Adults of Oil based Depot and other Long Acting Intramuscular Antipsychotic Injections Summary of Product Characteristics for: Clopixol (Zuclopethixol Decanoate) Flupenthixol Decanoate Haldol (Haloperidol Decanoate) Risperdal Consta Xeplion (Paliperidone Palmitate) ZypAdhera (Olanzapine Embonate) Abilify Maintena (Aripiprazole) 1 CONTENTS REFERENCES/KEY DOCUMENTS …………………………………………………………… 1 INTRODUCTION …………………………………………………………………………………. 3 SCOPE …………………………………………………………………………………………….. 3 DEFINITIONS ……………………………………………………………………………………... 3 CHOICE OF DRUG AND DOSAGE SELECTION ……………………………………………. 3 SIDE EFFECTS ……………………………………………………………….………………….. 5 PRESCRIBING …………………………………………………………………………………… 5 ADMINISTRATION ………………………………………………………………………………. 7 RECORDING ADMINISTRATION ……………………………………………………………… 7 -

Deanxit SPC.Pdf
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deanxit film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Flupentixol 0.5 mg (as 0.584 mg flupentixol dihydrochloride) Melitracen 10 mg (as 11.25 mg melitracen hydrochloride) Excipients with known effect: Lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablets. Round, biconvex, cyclamen, film-coated tablets. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Anxiety Depression Asthenia. Neurasthenia. Psychogenic depression. Depressive neuroses. Masked depression. Psychosomatic affections accompanied by anxiety and apathy. Menopausal depressions. Dysphoria and depression in alcoholics and drug-addicts. 4.2 Posology and method of administration Posology Adults Usually 2 tablets daily: morning and noon. In severe cases the morning dose may be increased to 2 tablets. The maximum dose is 4 tablets daily. Older people (> 65 years) 1 tablet in the morning. In severe cases 1 tablet in the morning and 1 at noon. Maintenance dose: Usually 1 tablet in the morning. SPC Portrait-REG_00051968 20v038 Page 1 of 10 In cases of insomnia or severe restlessness additional treatment with a sedative in the acute phase is recommended. Paediatric population Children and adolescents (<18 years) Deanxit is not recommended for use in children and adolescents due to lack of data on safety and efficacy. Reduced renal function Deanxit can be given in the recommended doses. Reduced liver function Deanxit can be given in the recommended doses. Method of administration The tablets are swallowed with water. 4.3 Contraindications Hypersensitivity to flupentixol and melitracen or to any of the excipients listed in section 6.1. -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Prescribing for Schizophrenia Guideline
CLINICAL GUIDELINE: Guidance for: Prescribing Guidance for the Treatment of No: Schizophrenia in Adults Agreed by: The Pharmacological Therapies Committee. January 2016 Trust Clinical Governance Committee. 2016 Aim of guideline The National Institute for Health and Care Excellence (NICE) published a guideline on the Treatment of Schizophrenia in Adults in February 2014. This guideline gives further advice on considerations when prescribing antipsychotics in Schizophrenia, including initial choice, monitoring and follow-up, as well as the approach to treatment-resistant Schizophrenia. Developed by Reaside Pharmacy Manager Consultant Psychiatrist Chief Pharmacist Consultant Psychiatrist Consultant Psychiatrist Consultant Psychiatrist Who it applies to This is prescribing guidance. Process for review / feedback Antipsychotic prescribing within the BSMHFT will be audited in a number of ways via the Pharmacological Therapies Committee for adherence to this guidance. This guidance will be updated annually in the light of any changes to any national or local prescribing practice. The next review date will be January 2018 1 Prescribing Guidance for the Treatment of Schizophrenia in Adults Primary care – make a referral to specialist mental health services Newly diagnosed On going Treatment resistant Should be seen by appropriate service Monitor mental state objectively to assess response to treatment. Offer clozapine if there is an as soon as possible regardless of age inadequate response to the If non-responsive despite increasing dose to maximum dose and Make full assessment and diagnosis sequential use of two different checking for side effects and adherence, then re-assess diagnosis antipsychotics, prescribed at and consider alternative medication Facilitate the choice of medication by adequate doses for a reasonable service user/carer by providing suitable length of time, at least one of Check medication adherence, using plasma assays if necessary.