Current P SYCHIATRY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aristada™ (Aripiprazole Lauroxil)
Aristada™ (aripiprazole lauroxil) – New Drug Approval • On October 5, 2015, Alkermes’ announced the FDA approval of Aristada (aripiprazole lauroxil) extended-release injection, an atypical antipsychotic, for the treatment of schizophrenia. • Schizophrenia is a chronic, severe and disabling brain disorder affecting an estimated 2.4 million Americans. Typically, symptoms include hearing voices, believing other people are reading their minds or controlling their thoughts, and being suspicious or withdrawn. • Aristada’s approval was based on data from a double-blind, placebo-controlled 12-week trial involving 622 patients with schizophrenia. In addition, the efficacy of Aristada was established, in part, on the basis of efficacy data from trials with oral aripiprazole. — Aristada significantly improved symptoms of schizophrenia compared to placebo at day 85. • Similar to other atypical antipsychotics, Aristada carries a boxed warning for increased mortality in elderly patients with dementia-related psychosis. • Other warnings and precautions for Aristada include cerebrovascular adverse reactions, including stroke; neuroleptic malignant syndrome; tardive dyskinesia; metabolic changes; orthostatic hypotension; leukopenia, neutropenia, and agranulocytosis; seizures; potential for cognitive and motor impairment; body temperature regulation; and dysphagia. • The most common adverse reaction (≥ 5% and at least twice that for placebo) with Aristada use was akathisia. • Aristada is administered by intramuscular injection in the deltoid (441 mg dose only) or gluteal (441 mg, 662 mg, or 882 mg) muscle by a healthcare professional. — Aristada can be initiated at a monthly dose (441 mg, 662 mg or 882 mg) or every 6 week dose (882 mg). — For patients naïve to aripiprazole, tolerability should be established with oral aripiprazole prior to initiating treatment with Aristada. -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

Investigation of the Cardiac Effects of Pancuronium, Rocuronium, Vecuronium, and Mivacurium on the Isolated Rat Atrium
Current Therapeutic Research VOLUME ,NUMBER ,OCTOBER Investigation of the Cardiac Effects of Pancuronium, Rocuronium, Vecuronium, and Mivacurium on the Isolated Rat Atrium Sinan Gursoy, MD1; Ihsan Bagcivan, MD2; Nedim Durmus, MD3; Kenan Kaygusuz, MD1; Iclal Ozdemir Kol, MD1; Cevdet Duger, MD1; Sahin Yildirim, MD2; and Caner Mimaroglu, MD1 1Department of Anesthesiology, Cumhuriyet University School of Medicine, Sivas, Turkey; 2Department of Pharmacology, Cumhuriyet University School of Medicine, Sivas, Turkey; and 3Ministry of Health of Turkey, General Directorate of Pharmacy and Pharmaceuticals, Ankara, Turkey ABSTRACT Background: Pancuronium, vecuronium, rocuronium, and mivacurium are nondepolarizing neuromuscular blocking agents that affect the cardiovascular system with different potencies. Their cardiovascular effects are clinically significant in the anesthetic management of patients, particularly those undergoing cardiac surgery. Objective: We aimed to compare the cardiac effects of these compounds, such as heart rate and developed force, in one species under identical experimental conditions in isolated rat atria. Methods: The left or right atria of rats were removed and suspended in organ baths. Pancuronium, vecuronium, rocuronium, or mivacurium were added cumula- tively (10–9–10–5 M) in the presence and absence of the nonselective -blocker propranolol (10–8 M) and the noradrenaline reuptake inhibitor desipramine (10–7 M), and heart rate changes were recorded in spontaneously beating right atria. Left atrial preparations were stimulated by electrical field stimulation using a bipolar platinum electrode, and the effects of cumulative concentrations of these nondepolarizing neuromuscular blocking agents on the developed force in the presence and absence of propranolol (10–8 M) and desipramine (10–7 M) were recorded. Results: Pancuronium increased heart rate in a dose-dependent manner com- pared with the control group (P Ͻ 0.027). -
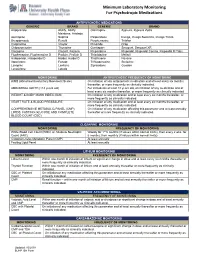
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Pharmacotherapy, Drug-Drug Interactions and Potentially
medRxiv preprint doi: https://doi.org/10.1101/2021.03.31.21254518; this version posted April 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Pharmacotherapy, drug-drug interactions and potentially inappropriate medication in depressive disorders Jan Wolff1,2,3, Pamela Reißner4, Gudrun Hefner5, Claus Normann2, Klaus Kaier6, Harald Binder6, Christoph Hiemke7, Sermin Toto8, Katharina Domschke2, Michael Marschollek1, Ansgar Klimke4,9 1 Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Germany. 2 Department of Psychiatry and Psychotherapy, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany. 3 Evangelical Foundation NeuerKerode, Germany. 4 Vitos Hochtaunus, Friedrichsdorf, Germany. 5 Vitos Clinic for Forensic Psychiatry, Eltville, Germany 6 Institute of Medical Biometry and Statistics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany. 7 Department of Psychiatry and Psychotherapy, University Medical Center Mainz, Germany. 8 Department of Psychiatry, Social Psychiatry and Psychotherapy, Hannover Medical School, Germany. 9 Heinrich-Heine-University Düsseldorf, Germany. ___ Correspondence Dr. Jan Wolff, Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Hannover, Germany. Address: Karl- Wiechert-Allee 3, 30625 Hannover. Email: [email protected], wolff.jan@mh- hannover.de, ORCID: https://orcid.org/0000-0003-2750-0606 Key words (MeSH) Depression, Polypharmacy, Antidepressants, Hospitals, Drug Interactions, Psychiatry NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Additional Antidepressant Pharmacotherapies According to A
orders & is T D h e n r Werner and Coveñas, Brain Disord Ther 2016, 5:1 i a a p r y B Brain Disorders & Therapy DOI: 10.4172/2168-975X.1000203 ISSN: 2168-975X Review Article Open Access Additional Antidepressant Pharmacotherapies According to a Neural Network Felix-Martin Werner1, 2* and Rafael Coveñas2 1Higher Vocational School of Elderly Care and Occupational Therapy, Euro Academy, Pößneck, Germany 2Laboratory of Neuroanatomy of the Peptidergic Systems, Institute of Neurosciences of Castilla y León (INCYL), University of Salamanca, Salamanca, Spain Abstract Major depression, a frequent psychiatric disease, is associated with neurotransmitter alterations in the midbrain, hypothalamus and hippocampus. Deficiency of postsynaptic excitatory neurotransmitters such as dopamine, noradrenaline and serotonin and a surplus of presynaptic inhibitory neurotransmitters such as GABA and glutamate (mainly a postsynaptic excitatory and partly a presynaptic inhibitory neurotransmitter), can be found in the involved brain regions. However, neuropeptide alterations (galanin, neuropeptide Y, substance P) also play an important role in its pathogenesis. A neural network is described, including the alterations of neuroactive substances at specific subreceptors. Currently, major depression is treated with monoamine reuptake inhibitors. An additional therapeutic option could be the administration of antagonists of presynaptic inhibitory neurotransmitters or the administration of agonists/antagonists of neuropeptides. Keywords: Acetylcholine; Bupropion; Dopamine; GABA; Galanin; in major depression and to point out the coherence between single Glutamate; Hippocampus; Hhypothalamus; Major depression; neuroactive substances and their corresponding subreceptors. A Midbrain; Neural network; Neuropeptide Y; Noradrenaline; Serotonin; question should be answered, whether a multimodal pharmacotherapy Substance P with an agonistic or antagonistic effect at several subreceptors is higher than the current conventional antidepressant treatment. -

Clinical Review, Adverse Events
Clinical Review, Adverse Events Drug: Carbamazepine NDA: 16-608, Tegretol 20-712, Carbatrol 21-710, Equetro Adverse Event: Stevens-Johnson Syndrome Reviewer: Ronald Farkas, MD, PhD Medical Reviewer, DNP, ODE I 1. Executive Summary 1.1 Background Carbamazepine (CBZ) is an anticonvulsant with FDA-approved indications in epilepsy, bipolar disorder and neuropathic pain. CBZ is associated with Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), closely related serious cutaneous adverse drug reactions that can be permanently disabling or fatal. Other anticonvulsants, including phenytoin, phenobarbital, and lamotrigine are also associated with SJS/TEN, as are members of a variety of other drug classes, including nonsteriodal anti-inflammatory drugs and sulfa drugs. The incidence of CBZ-associated SJS/TEN has been considered “extremely rare,” as noted in current U.S. drug labeling. However, recent publications and postmarketing data suggest that CBZ- associated SJS/TEN occurs at a much higher rate in some Asian populations, about 2.5 cases per 1,000 new exposures, and that most of this increased risk is in individuals carrying a specific human leukocyte antigen (HLA) allele, HLA-B*1502. This HLA-B allele is present in about 5- to 20% of many, but not all, Asian populations, and is also present in about 2- to 4% of South Asians/Indians. The allele is also present at a lower frequency, < 1%, in several other ethnic groups around the world (although likely due to distant Asian ancestry). About 10% of U.S. Asians carry HLA-B*1502. HLA-B*1502 is generally not present in the U.S. -

Hyponatremia and Psychotropic Drugs
DOI: 10.5772/intechopen.79029 ProvisionalChapter chapter 2 Hyponatremia and Psychotropic Drugs MireiaMireia Martínez Cortés Martínez Cortés and Pedro Gurillo MuñozPedro Gurillo Muñoz Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.79029 Abstract Given the widespread use of psychotropic drugs in the population, it’s important to consider hyponatremia as an avoidable and reversible adverse effect and include the detection of high-risk subjects to establish safer medications, as well as early detection measures in routine clinical practice. Although hyponatremia has been especially associ- ated with serotonergic antidepressants (SSRIs), there is also an elevated risk with tricy- clics, duals and heterocyclic antidepressants, due to the different mechanisms of action at the renal tubular level and the release of ADH. Hyponatremia secondary to tricyclics with slow CYP2D6 metabolizers have higher plasma concentrations of antidepressants metabolized by CYP2D6. Hyponatremia secondary to SSRIs appears in the first week of treatment, it is “not dose-dependent” and normalization of natremia occurs between 2 and 20 days after stopping the medication. Bupropion, trazodone, mianserin, reboxetine and agomelatine are a safe alternative. Also antiepileptics have been related to hypona- tremia. Both typical and atypical antipsychotics have been exposed to an increased risk of hyponatremia, even after adjusted factors such as age, sex and comorbidity. Other factors that favor the onset of hyponatremia act synergistically with psychotropic drugs, such as: advanced age, female sex, concomitant diuretic intake, low body weight and low sodium levels; NSAID, ACEIs, and warm. Keywords: hyponatremia, antipsychotic, antidepressant, antiepileptic, psychotropic drugs 1. Introduction Hyponatremia is the most frequent hydroelectrolytic disorder in clinical practice, both in hospital and outpatient settings [1]. -

Antipsychotic Combinations
Graylands Hospital DRUG BULLETIN Pharmacy Department Brockway Road Mount Claremont WA 6010 Telephone (08) 9347 6400 Email [email protected] Fax (08) 9384 4586 Antipsychotic Combinations Graylands Hospital Drug Bulletin 2008 Vol. 15 No. 3 February ISSN 1323-1251 Antipsychotic combinations Reasons for antipsychotic combinations Despite the development of efficacious medications for the treatment of schizophrenia, many people do There are a number of theoretical benefits and not respond adequately. To address this problem, reasons cited for antipsychotic combination the use of two or more antipsychotics simultaneously prescribing, these include: is a commonly employed treatment strategy. Although the use of combination antipsychotics is Complementary mechanisms of action4 (e.g. common in clinical practice, the risks and benefits adding an antipsychotic with strong dopamine have not been systematically evaluated to date. As a D2 blockade to a weak D2 blocker) result, current Australian treatment algorithms Partial replacement of antipsychotic action for including the Royal Australian and New Zealand drugs with intolerable adverse effects at higher College of Psychiatry Schizophrenia Guidelines and 5 doses (e.g. adding quetiapine to clozapine to the Western Australian Therapeutic Advisory Group minimise metabolic adverse effects) Antipsychotic Guidelines advise against the use of combined antipsychotics, except for short periods of Alternative where clozapine cannot be used6 changeover1,2. Most data on antipsychotic -

Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice
pharmaceuticals Article Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice Geng-Ruei Chang 1,* , Po-Hsun Hou 2,3, Wei-Cheng Yang 4, Chao-Min Wang 1 , Pei-Shan Fan 1, Huei-Jyuan Liao 1 and To-Pang Chen 5,* 1 Department of Veterinary Medicine, National Chiayi University, 580 Xinmin Road, Chiayi 60054, Taiwan; [email protected] (C.-M.W.); [email protected] (P.-S.F.); [email protected] (H.-J.L.) 2 Department of Psychiatry, Taichung Veterans General Hospital, 1650 Taiwan Boulevard (Section 4), Taichung 40705, Taiwan; [email protected] 3 Faculty of Medicine, National Yang-Ming University, 155 Linong Street (Section 2), Taipei 11221, Taiwan 4 School of Veterinary Medicine, National Taiwan University, 1 Roosevelt Road (Section 4), Taipei 10617, Taiwan; [email protected] 5 Division of Endocrinology and Metabolism, Show Chwan Memorial Hospital, 542 Chung-Shan Road (Section 1), Changhua 50008, Taiwan * Correspondence: [email protected] (G.-R.C.); [email protected] (T.-P.C.); Tel.: +886-5-2732946 (G.-R.C.); +886-4-7256166 (T.-P.C.) Abstract: Doxepin is commonly prescribed for depression and anxiety treatment. Doxepin-related disruptions to metabolism and renal/hepatic adverse effects remain unclear; thus, the underlying mechanism of action warrants further research. Here, we investigated how doxepin affects lipid Citation: Chang, G.-R.; Hou, P.-H.; change, glucose homeostasis, chromium (Cr) distribution, renal impairment, liver damage, and fatty Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; liver scores in C57BL6/J mice subjected to a high-fat diet and 5 mg/kg/day doxepin treatment for Liao, H.-J.; Chen, T.-P. -

Olanzapine-Induced Acute Pancreatitis and New Diabetes Mellitus
Open Journal of Psychiatry, 2012, 2, 110-112 OJPsych http://dx.doi.org/10.4236/ojpsych.2012.22015 Published Online April 2012 (http://www.SciRP.org/journal/ojpsych/) Case report: Olanzapine-induced acute pancreatitis and new diabetes mellitus Erik Monasterio1*, Ruchi Bhalla2, Andrew McKean3 1Department of Psychological Medicine, University of Otago, Christchurch, New Zealand 2Hammersmith and Fulham Mental Health Unit, West London Mental Health NHS Trust, London, UK 3Hillmorton Hospital, Christchurch, New Zealand Email: *[email protected] Received 29 December 2011; revised 31 January 2012; accepted 15 February 2012 ABSTRACT criteria and required application from a psychiatrist for a patient who had been trialled unsuccessfully on risperi- The aim of this case study is to review the literature done or required treatment with olanzapine short acting and report the first published case of olanzapine-in- intra-muscular injection [4]. In June 2011, significantly duced acute pancreatitis in New Zealand. A case re- cheaper generic versions of olanzapine were introduced port of acute pancreatitis with new onset diabetes and the special authority criteria for olanzapine with- mellitus secondary to olanzapine in a 42-year-old male, drawn [5]. We aim to highlight lesser known and poten- in the absence of medical risk factors is reported. tially fatal side effects through our case report. Eleven previous case reports of olanzapine induced acute-pancreatitis were identified in the literature. A 2. CASE REPORT 42-year-old male was diagnosed with acute pancreati- tis and new diabetes mellitus induced by olanzapine. Mr. X is a 42 year old man who had come into contact Although rare, pancreatitis is associated with use of with mental health services on many occasions during some atypical antipsychotic medications.