Long-Acting Injectable Antipsychotics: Haloperidol and Fluphenazine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aripiprazole As an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines
TITLE: Aripiprazole as an Adjunct to Anti-Depressants for Major Depressive Disorder: Clinical Effectiveness, Cost-Effectiveness, and Guidelines DATE: 04 May 2016 RESEARCH QUESTIONS 1. What is the clinical efficacy of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 2. What is the cost-effectiveness of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? 3. What are the evidence-based guidelines for the use of aripiprazole for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments? KEY FINDINGS Three systematic reviews with meta-analyses, two randomized controlled trials, and two evidence-based guidelines were identified regarding the use of aripiprazole as an adjunct to antidepressants for the treatment of patients with major depressive disorder who have had an inadequate response to prior antidepressant treatments. METHODS A limited literature search was conducted on key resources including PubMed, Ovid PsychINFO, Ovid Embase, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No methodological filters were used to limit retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and April 29, 2016. Disclaimer: The Rapid Response Service is an information service for those involved in planning and providing health care in Canada. -

HALDOL Brand of Haloperidol Injection (For Immediate Release) WARNING Increased Mortality in Elderly Patients with Dementia
HALDOL® brand of haloperidol injection (For Immediate Release) WARNING Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. HALDOL Injection is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS). DESCRIPTION Haloperidol is the first of the butyrophenone series of major antipsychotics. The chemical designation is 4-[4-(p-chlorophenyl)-4-hydroxypiperidino] 4’-fluorobutyrophenone and it has the following structural formula: HALDOL (haloperidol) is available as a sterile parenteral form for intramuscular injection. The injection provides 5 mg haloperidol (as the lactate) and lactic acid for pH adjustment between 3.0 – 3.6. -

ALEX COLOMÉ (48) COLOMÉ ALEX Tommy Romero (Rhp), May 25, 2018
ALEX COLOMÉ (48) POSITION: Right-Handed Pitcher AGE: 29 BORN: 12-31-88 in Santo Domingo, DR BATS: Right THROWS: Right HEIGHT: 6-1 WEIGHT: 220 ML SERVICE: 3 years, 118 days CONTRACT STATUS: Signed through 2018 ACQUIRED: In trade with Tampa Bay along with Denard Span (of) 2018 MARINERS and cash considerations in exchange for Andrew Moore (rhp) and Tommy Romero (rhp), May 25, 2018. PRONUNCIATION: Colomé (COLE-uh-may) 2017: • The Totals – Went 2-3 with 47 saves COLOMÉ’s CAREER HIGHS and a 3.24 ERA (24 ER, 66.2 IP) with MOST STRIKEOUTS: 58 strikeouts and 23 walks in 65 relief STARTER: 7 – 5/30/13 at MIA w/TB appearances with Tampa Bay. RELIEVER: 4 — 7/26/15 vs. BAL w/TB • Leader – Became the first pitcher LOW-HIT GAME: None in club history to lead the Major LONGEST WINNING STREAK: Leagues in saves…his 47 saves were 4 – 6/27/14 – 5/6/15 w/TB one shy of the club record, set by LONGEST LOSING STREAK: Fernando Rodney in 2012 (48)…his 4 – 4/15 – 8/26/16 w/TB 47 saves were 6 more than any other pitcher in the Majors (Greg Holland- MOST INNINGS: COL and Kenley Jansen-LAD) and 8 STARTER: 7.0 – 2 times, more than any other pitcher in the AL last: 7/1/15 vs. CLE w/TB (Roberto Osuna-TOR). RELIEVER: 4.0 – 5/26/14 at TOR w/TB • Length – Led the American League with 6 saves of 4 outs or more. • Award Season – Named American League Reliever of the Month for August…was 10- for-10 in save opportunities while posting a 0.75 ERA (1 ER, 12.0 IP) with 13 strikeouts and 1 walk in 12 games. -

Supplement of Hydrol
Supplement of Hydrol. Earth Syst. Sci., 25, 957–982, 2021 https://doi.org/10.5194/hess-25-957-2021-supplement © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement of Learning from satellite observations: increased understanding of catchment processes through stepwise model improvement Petra Hulsman et al. Correspondence to: Petra Hulsman ([email protected]) The copyright of individual parts of the supplement might differ from the CC BY 4.0 License. Supplements S1. Model performance with respect to all discharge signatures ............................................... 2 S2. Parameter sets selected based on discharge ......................................................................... 3 S2.1 Time series: Discharge ............................................................................................................................... 3 S2.2. Time series: Evaporation (Basin average) ................................................................................................. 4 S2.3 Time series: Evaporation (Wetland dominated areas) ................................................................................ 5 S2.4 Time series: Total water storage (Basin average) ....................................................................................... 6 S2.5. Spatial pattern: Evaporation (normalised, dry season) .............................................................................. 7 S2.6. Spatial pattern: Total water storage (normalised, dry season) .................................................................. -

Treatment of Psychosis: 30 Years of Progress
Journal of Clinical Pharmacy and Therapeutics (2006) 31, 523–534 REVIEW ARTICLE Treatment of psychosis: 30 years of progress I. R. De Oliveira* MD PhD andM.F.Juruena MD *Department of Neuropsychiatry, School of Medicine, Federal University of Bahia, Salvador, BA, Brazil and Department of Psychological Medicine, Institute of Psychiatry, King’s College, University of London, London, UK phrenia. Thirty years ago, psychiatrists had few SUMMARY neuroleptics available to them. These were all Background: Thirty years ago, psychiatrists had compounds, today known as conventional anti- only a few choices of old neuroleptics available to psychotics, and all were liable to cause severe extra them, currently defined as conventional or typical pyramidal side-effects (EPS). Nowadays, new antipsychotics, as a result schizophrenics had to treatments are more ambitious, aiming not only to suffer the severe extra pyramidal side effects. improve psychotic symptoms, but also quality of Nowadays, new treatments are more ambitious, life and social reinsertion. We briefly but critically aiming not only to improve psychotic symptoms, outline the advances in diagnosis and treatment but also quality of life and social reinsertion. Our of schizophrenia, from the mid 1970s up to the objective is to briefly but critically review the present. advances in the treatment of schizophrenia with antipsychotics in the past 30 years. We conclude DIAGNOSIS OF SCHIZOPHRENIA that conventional antipsychotics still have a place when just the cost of treatment, a key factor in Up until the early 1970s, schizophrenia diagnoses poor regions, is considered. The atypical anti- remained debatable. The lack of uniform diagnostic psychotic drugs are a class of agents that have criteria led to relative rates of schizophrenia being become the most widely used to treat a variety of very different, for example, in New York and psychoses because of their superiority with London, as demonstrated in an important study regard to extra pyramidal symptoms. -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

Download Executive Summary
Comparative Effectiveness Review Number 43 Effective Health Care Program Off-Label Use of Atypical Antipsychotics: An Update Executive Summary Background Effective Health Care Program Antipsychotics medications are approved The Effective Health Care Program by the U.S. Food and Drug Administration was initiated in 2005 to provide valid (FDA) for treatment of schizophrenia and evidence about the comparative bipolar disorder. These medications are effectiveness of different medical commonly divided into two classes, interventions. The object is to help reflecting two waves of historical consumers, health care providers, and development: the conventional others in making informed choices antipsychotics and the atypical. The among treatment alternatives. Through conventional antipsychotics served as the its Comparative Effectiveness Reviews, first successful pharmacologic treatment the program supports systematic for primary psychotic disorders such as appraisals of existing scientific schizophrenia. Having been widely used evidence regarding treatments for for decades, the conventional high-priority health conditions. It also antipsychotics also produced various side promotes and generates new scientific effects requiring additional medications, evidence by identifying gaps in which spurred the development of the existing scientific evidence and atypical antipsychotics. supporting new research. The program Currently, nine atypical antipsychotic drugs puts special emphasis on translating have been approved by FDA: aripiprazole, findings into a variety of useful asenapine, clozapine, iloperidone, formats for different stakeholders, olanzapine, paliperidone, quetiapine, including consumers. risperidone, and ziprasidone. These drugs The full report and this summary are have been used off-label (i.e., for available at www.effectivehealthcare. indications not approved by FDA) for the ahrq.gov/reports/final.cfm. treatment of various psychiatric conditions. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Medication Conversion Chart
Fluphenazine FREQUENCY CONVERSION RATIO ROUTE USUAL DOSE (Range) (Range) OTHER INFORMATION KINETICS Prolixin® PO to IM Oral PO 2.5-20 mg/dy QD - QID NA ↑ dose by 2.5mg/dy Q week. After symptoms controlled, slowly ↓ dose to 1-5mg/dy (dosed QD) Onset: ≤ 1hr 1mg (2-60 mg/dy) Caution for doses > 20mg/dy (↑ risk EPS) Cmax: 0.5hr 2.5mg Elderly: Initial dose = 1 - 2.5mg/dy t½: 14.7-15.3hr 5mg Oral Soln: Dilute in 2oz water, tomato or fruit juice, milk, or uncaffeinated carbonated drinks Duration of Action: 6-8hr 10mg Avoid caffeinated drinks (coffee, cola), tannics (tea), or pectinates (apple juice) 2° possible incompatibilityElimination: Hepatic to inactive metabolites 5mg/ml soln Hemodialysis: Not dialyzable HCl IM 2.5-10 mg/dy Q6-8 hr 1/3-1/2 po dose = IM dose Initial dose (usual): 1.25mg Onset: ≤ 1hr Immediate Caution for doses > 10mg/dy Cmax: 1.5-2hr Release t½: 14.7-15.3hr 2.5mg/ml Duration Action: 6-8hr Elimination: Hepatic to inactive metabolites Hemodialysis: Not dialyzable Decanoate IM 12.5-50mg Q2-3 wks 10mg po = 12.5mg IM CONVERTING FROM PO TO LONG-ACTING DECANOATE: Onset: 24-72hr (4-72hr) Long-Acting SC (12.5-100mg) (1-4 wks) Round to nearest 12.5mg Method 1: 1.25 X po daily dose = equiv decanoate dose; admin Q2-3wks. Cont ½ po daily dose X 1st few mths Cmax: 48-96hr 25mg/ml Method 2: ↑ decanoate dose over 4wks & ↓ po dose over 4-8wks as follows (accelerate taper for sx of EPS): t½: 6.8-9.6dy (single dose) ORAL DECANOATE (Administer Q 2 weeks) 15dy (14-100dy chronic administration) ORAL DOSE (mg/dy) ↓ DOSE OVER (wks) INITIAL DOSE (mg) TARGET DOSE (mg) DOSE OVER (wks) Steady State: 2mth (1.5-3mth) 5 4 6.25 6.25 0 Duration Action: 2wk (1-6wk) Elimination: Hepatic to inactive metabolites 10 4 6.25 12.5 4 Hemodialysis: Not dialyzable 20 8 6.25 12.5 4 30 8 6.25 25 4 40 8 6.25 25 4 Method 3: Admin equivalent decanoate dose Q2-3wks. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -
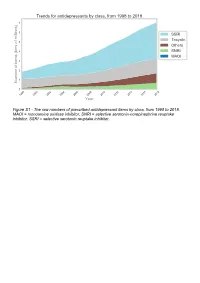
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Amisulpride Tablets I.P. SOLIAN® THERAPEUTIC CATEGORY Anti-Psychotic COMPOSITION Solian® 50 /100 /200 /400 Each Uncoated Tablet Contains Amisulpride IP
For the use only of a Registered Medical Practitioner (Psychiatrist) or a Hospital or a Laboratory Abridged Prescribing Information Amisulpride tablets I.P. SOLIAN® THERAPEUTIC CATEGORY Anti-psychotic COMPOSITION Solian® 50 /100 /200 /400 Each uncoated tablet contains Amisulpride IP. 50mg / 100mg / 200mg Each film coated tablet contains Amisulpride IP 400mg. THERAPEUTIC INDICATIONS Treatment of acute and chronic schizophrenic disorders, in which positive symptoms (such as delusions, hallucinations, and thought disorders) and/or negative symptoms (such as blunted affect, emotional and social withdrawal) are prominent, including patients characterised by predominant negative symptoms. DOSAGE AND ADMINISTRATION For acute psychotic episodes, oral doses between 400 and 800 mg/d are recommended. Doses above 1200 mg/d should not be used. For patients with mixed positive and negative symptoms, doses should be adjusted to obtain optimal control of positive symptoms. Maintenance treatment should be established individually with the minimally effective dose. For patients characterised by predominant negative symptoms, oral doses between 50 mg/d and 300 mg/d are recommended. Doses should be adjusted individually. Solian® can be administered once daily at oral doses up to 300 mg, higher doses should be administered bid. The Minimum effective dose should be used. Caution in elderly. Renal & Hepatic insufficiency: Dose should be reduced. Use of amisulpride from puberty to 18 years is not recommended. SAFETY-RELATED INFORMATION Contraindications: Hypersensitivity to amisulpride or to other ingredients of the product; concomitant prolactin- dependent tumours e.g. pituitary gland prolactinomas and breast cancer; phaeochromocytoma; children up to puberty; lactation; combinations with drugs which could induce torsades de pointes and levodopa.