Informationen Zur
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
HIGHLIGHTS OF PRESCRIBING INFORMATION • Metabolic Changes: Atypical antipsychotic drugs have been associated with These highlights do not include all the information needed to use metabolic changes that may increase cardiovascular/cerebrovascular risk. CLOZARIL safely and effectively. See full prescribing information for These metabolic changes include: CLOZARIL. o Hyperglycemia and Diabetes Mellitus: Monitor for symptoms of CLOZARIL® (clozapine) tablets, for oral use hyperglycemia including polydipsia, polyuria, polyphagia, and Initial U.S. Approval: 1989 weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes. (5.9) WARNING: SEVERE NEUTROPENIA; ORTHOSTATIC o Dyslipidemia: Undesirable alterations in lipids have occurred in HYPOTENSION, BRADYCARDIA, AND SYNCOPE; SEIZURE; patients treated with atypical antipsychotics. (5.9) MYOCARDITIS AND CARDIOMYOPATHY; INCREASED o Weight Gain: Significant weight gain has occurred. Monitor weight MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA- gain. (5.9) RELATED PSYCHOSIS • Neuroleptic Malignant Syndrome (NMS): Immediately discontinue and See full prescribing information for complete boxed warning. monitor closely. Assess for co-morbid conditions. (5.10) • Fever: Evaluate for infection and for neutropenia, NMS. (5.11) • Pulmonary Embolism (PE): Consider PE if respiratory distress, chest pain, • Severe Neutropenia: CLOZARIL can cause severe neutropenia, which or deep-vein thrombosis occur. (5.12) can lead to serious and fatal infections. Patients initiating and • Anticholinergic Toxicity: Use cautiously in presence of specific conditions continuing treatment with CLOZARIL must have a baseline blood (e.g., narrow angle glaucoma, use of anticholinergic drugs). (5.13) absolute neutrophil count (ANC) measured before treatment initiation • Interference with Cognitive and Motor Performance: Advise caution when and regular ANC monitoring during treatment (2.1, 5.1). -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Management of Side Effects of Antipsychotics
Management of side effects of antipsychotics Oliver Freudenreich, MD, FACLP Co-Director, MGH Schizophrenia Program www.mghcme.org Disclosures I have the following relevant financial relationship with a commercial interest to disclose (recipient SELF; content SCHIZOPHRENIA): • Alkermes – Consultant honoraria (Advisory Board) • Avanir – Research grant (to institution) • Janssen – Research grant (to institution), consultant honoraria (Advisory Board) • Neurocrine – Consultant honoraria (Advisory Board) • Novartis – Consultant honoraria • Otsuka – Research grant (to institution) • Roche – Consultant honoraria • Saladax – Research grant (to institution) • Elsevier – Honoraria (medical editing) • Global Medical Education – Honoraria (CME speaker and content developer) • Medscape – Honoraria (CME speaker) • Wolters-Kluwer – Royalties (content developer) • UpToDate – Royalties, honoraria (content developer and editor) • American Psychiatric Association – Consultant honoraria (SMI Adviser) www.mghcme.org Outline • Antipsychotic side effect summary • Critical side effect management – NMS – Cardiac side effects – Gastrointestinal side effects – Clozapine black box warnings • Routine side effect management – Metabolic side effects – Motor side effects – Prolactin elevation • The man-in-the-arena algorithm www.mghcme.org Receptor profile and side effects • Alpha-1 – Hypotension: slow titration • Dopamine-2 – Dystonia: prophylactic anticholinergic – Akathisia, parkinsonism, tardive dyskinesia – Hyperprolactinemia • Histamine-1 – Sedation – Weight gain -

Appendix 13C: Clinical Evidence Study Characteristics Tables
APPENDIX 13C: CLINICAL EVIDENCE STUDY CHARACTERISTICS TABLES: PHARMACOLOGICAL INTERVENTIONS Abbreviations ............................................................................................................ 3 APPENDIX 13C (I): INCLUDED STUDIES FOR INITIAL TREATMENT WITH ANTIPSYCHOTIC MEDICATION .................................. 4 ARANGO2009 .................................................................................................................................. 4 BERGER2008 .................................................................................................................................... 6 LIEBERMAN2003 ............................................................................................................................ 8 MCEVOY2007 ................................................................................................................................ 10 ROBINSON2006 ............................................................................................................................. 12 SCHOOLER2005 ............................................................................................................................ 14 SIKICH2008 .................................................................................................................................... 16 SWADI2010..................................................................................................................................... 19 VANBRUGGEN2003 .................................................................................................................... -
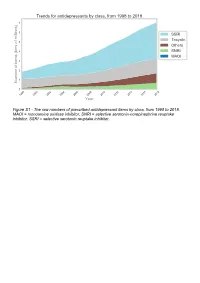
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

FDA Approved Drugs with Broad Anti-Coronaviral Activity Inhibit SARS-Cov-2 in Vitro
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.25.008482; this version posted March 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro Stuart Weston1, Rob Haupt1, James Logue1, Krystal Matthews1 and Matthew B. Frieman*1 1 - Department of Microbiology and Immunology, University of Maryland School of Medicine, 685 W. Baltimore St., Room 380, Baltimore, MD, 21201, USA *Corresponding author. Email: [email protected] Key words: SARS-CoV-2, nCoV-2019, COVID-19, drug repurposing, FDA approved drugs, antiviral therapeutics, pandemic, chloroquine, hydroxychloroquine AbstraCt SARS-CoV-2 emerged in China at the end of 2019 and has rapidly become a pandemic with over 400,000 recorded COVID-19 cases and greater than 19,000 recorded deaths by March 24th, 2020 (www.WHO.org) (1). There are no FDA approved antivirals or vaccines for any coronavirus, including SARS-CoV-2 (2). Current treatments for COVID-19 are limited to supportive therapies and off-label use of FDA approved drugs (3). Rapid development and human testing of potential antivirals is greatly needed. A potentially quicker way to test compounds with antiviral activity is through drug re-purposing (2, 4). Numerous drugs are already approved for use in humans and subsequently there is a good understanding of their safety profiles and potential side effects, making them easier to test in COVID-19 patients. -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Revision of Precautions Asenapine Maleate, Aripiprazole, Olanzapine
Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Revision of Precautions Asenapine maleate, aripiprazole, olanzapine, quetiapine fumarate, clocapramine hydrochloride hydrate, chlorpromazine hydrochloride, chlorpromazine hydrochloride/promethazine hydrochloride/phenobarbital, chlorpromazine phenolphthalinate, spiperone, zotepine, timiperone, haloperidol, paliperidone, pipamperone hydrochloride, fluphenazine decanoate, fluphenazine maleate, brexpiprazole, prochlorperazine maleate, prochlorperazine mesilate, Pharmaceuticals and Medical Devices Agency Office of Safety I 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan E-mail: [email protected] Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. propericiazine, bromperidol, perphenazine, perphenazine hydrochloride, perphenazine fendizoate, perphenazine maleate, perospirone hydrochloride hydrate, mosapramine hydrochloride, risperidone (oral drug), levomepromazine hydrochloride, levomepromazine maleate March 27, 2018 Non-proprietary name Asenapine maleate, -

Download Product Insert (PDF)
PRODUCT INFORMATION Chlorprothixene (hydrochloride) Item No. 20772 CAS Registry No.: 6469-93-8 Formal Name: (3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N- N dimethyl-1-propanamine, monohydrochloride Synonyms: cis-Chlorprothixene, NSC 169899 MF: C18H18ClNS • HCl Cl FW: 352.3 Purity: ≥98% UV/Vis.: λmax: 230, 270, 329 nm S Supplied as: A crystalline solid • HCl Storage: -20°C Stability: As supplied, 2 years from the QC date provided on the Certificate of Analysis, when stored properly Laboratory Procedures Chlorprothixene (hydrochloride) is supplied as a crystalline solid. A stock solution may be made by dissolving the chlorprothixene (hydrochloride) in the solvent of choice. Chlorprothixene (hydrochloride) is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of chlorprothixene (hydrochloride) in these solvents is approximately 30 mg/ml. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of chlorprothixene (hydrochloride) can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of chlorprothixene (hydrochloride) in PBS, pH 7.2, is approximately 10 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description 1 Chlorprothixene is a thioxanthine antipsychotic that functions by antagonizing dopamine D2 receptors. It 2 can block a subset of GABAA receptors in rat cortex that is also blocked by clozapine (Item No. -

Antipsychotics and the Risk of Sudden Cardiac Death
ORIGINAL INVESTIGATION Antipsychotics and the Risk of Sudden Cardiac Death Sabine M. J. M. Straus, MD; Gyse`le S. Bleumink, MD; Jeanne P. Dieleman, PhD; Johan van der Lei, MD, PhD; Geert W. ‘t Jong, PhD; J. Herre Kingma, MD, PhD; Miriam C. J. M. Sturkenboom, PhD; Bruno H. C. Stricker, PhD Background: Antipsychotics have been associated with Results: The study population comprised 554 cases of prolongation of the corrected QT interval and sudden car- sudden cardiac death. Current use of antipsychotics was diac death. Only a few epidemiological studies have in- associated with a 3-fold increase in risk of sudden car- vestigated this association. We performed a case- diac death. The risk of sudden cardiac death was high- control study to investigate the association between use est among those using butyrophenone antipsychotics, of antipsychotics and sudden cardiac death in a well- those with a defined daily dose equivalent of more than defined community-dwelling population. 0.5 and short-term (Յ90 days) users. The association with current antipsychotic use was higher for witnessed cases Methods: We performed a population-based case-control (n=334) than for unwitnessed cases. study in the Integrated Primary Care Information (IPCI) project, a longitudinal observational database with com- Conclusions: Current use of antipsychotics in a gen- plete medical records from 150 general practitioners. All eral population is associated with an increased risk of sud- instances of death between January 1, 1995, and April 1, den cardiac death, even at a low dose and for indica- 2001, were reviewed. Sudden cardiac death was classified tions other than schizophrenia.