Should Inverse Agonists Be Defined by Pharmacological Mechanism Or
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

ZANTAC® 150 (Ranitidine Hydrochloride) Tablets, USP
PRESCRIBING INFORMATION ZANTAC® 150 (ranitidine hydrochloride) Tablets, USP ZANTAC® 300 (ranitidine hydrochloride) Tablets, USP ZANTAC® 25 (ranitidine hydrochloride effervescent) ® EFFERdose Tablets ® ZANTAC (ranitidine hydrochloride) Syrup, USP DESCRIPTION The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, ZANTAC 25 EFFERdose Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2 furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structure: The empirical formula is C13H22N4O3S•HCl, representing a molecular weight of 350.87. Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfurlike odor. Each ZANTAC 150 Tablet for oral administration contains 168 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients FD&C Yellow No. 6 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, triacetin, and yellow iron oxide. Each ZANTAC 300 Tablet for oral administration contains 336 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredients croscarmellose sodium, D&C Yellow No. 10 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin. 1 ZANTAC 25 EFFERdose Tablets for oral administration is an effervescent formulation of ranitidine that must be dissolved in water before use. Each individual tablet contains 28 mg of ranitidine HCl equivalent to 25 mg of ranitidine and the following inactive ingredients: aspartame, monosodium citrate anhydrous, povidone, and sodium bicarbonate. Each tablet also contains sodium benzoate. -

2D6 Substrates 2D6 Inhibitors 2D6 Inducers
Physician Guidelines: Drugs Metabolized by Cytochrome P450’s 1 2D6 Substrates Acetaminophen Captopril Dextroamphetamine Fluphenazine Methoxyphenamine Paroxetine Tacrine Ajmaline Carteolol Dextromethorphan Fluvoxamine Metoclopramide Perhexiline Tamoxifen Alprenolol Carvedilol Diazinon Galantamine Metoprolol Perphenazine Tamsulosin Amiflamine Cevimeline Dihydrocodeine Guanoxan Mexiletine Phenacetin Thioridazine Amitriptyline Chloropromazine Diltiazem Haloperidol Mianserin Phenformin Timolol Amphetamine Chlorpheniramine Diprafenone Hydrocodone Minaprine Procainamide Tolterodine Amprenavir Chlorpyrifos Dolasetron Ibogaine Mirtazapine Promethazine Tradodone Aprindine Cinnarizine Donepezil Iloperidone Nefazodone Propafenone Tramadol Aripiprazole Citalopram Doxepin Imipramine Nifedipine Propranolol Trimipramine Atomoxetine Clomipramine Encainide Indoramin Nisoldipine Quanoxan Tropisetron Benztropine Clozapine Ethylmorphine Lidocaine Norcodeine Quetiapine Venlafaxine Bisoprolol Codeine Ezlopitant Loratidine Nortriptyline Ranitidine Verapamil Brofaramine Debrisoquine Flecainide Maprotline olanzapine Remoxipride Zotepine Bufuralol Delavirdine Flunarizine Mequitazine Ondansetron Risperidone Zuclopenthixol Bunitrolol Desipramine Fluoxetine Methadone Oxycodone Sertraline Butylamphetamine Dexfenfluramine Fluperlapine Methamphetamine Parathion Sparteine 2D6 Inhibitors Ajmaline Chlorpromazine Diphenhydramine Indinavir Mibefradil Pimozide Terfenadine Amiodarone Cimetidine Doxorubicin Lasoprazole Moclobemide Quinidine Thioridazine Amitriptyline Cisapride -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients Research Questions: 1. How many schizophrenia patients are prescribed recommended first-line second-generation treatments for schizophrenia? 2. How many schizophrenia patients switch to an injectable antipsychotic after stabilization on an oral antipsychotic? 3. How many schizophrenia patients are prescribed 2 or more concomitant antipsychotics? 4. Are claims for long-acting injectable antipsychotics primarily billed as pharmacy or physician administered claims? 5. Does adherence to antipsychotic therapy differ between patients with claims for different routes of administration (oral vs. long-acting injectable)? Conclusions: In total, 4663 schizophrenia patients met inclusion criteria, and approximately 14% of patients (n=685) were identified as treatment naïve without claims for antipsychotics in the year before their first antipsychotic prescription. Approximately 45% of patients identified as treatment naïve had a history of remote antipsychotic use, but it is unclear if antipsychotics were historically prescribed for schizophrenia. Oral second-generation antipsychotics which are recommended as first-line treatment in the MHCAG schizophrenia algorithm were prescribed as initial treatment in 37% of treatment naive patients and 28% of all schizophrenia patients. Recommended agents include risperidone, paliperidone, and aripiprazole. Utilization of parenteral antipsychotics was limited in patients with schizophrenia. Overall only 8% of patients switched from an oral to an injectable therapy within 6 months of their first claim. Approximately, 60% of all schizophrenia patients (n=2512) had claims for a single antipsychotic for at least 12 continuous weeks and may be eligible to transition to a long-acting injectable antipsychotic. -
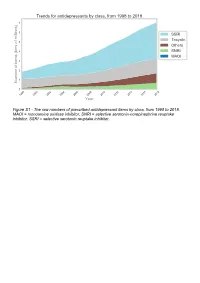
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Receptor Antagonist (H RA) Shortages | May 25, 2020 2 2 2 GERD4,5 • Take This Opportunity to Determine If Continued Treatment Is Necessary
H2-receptor antagonist (H2RA) Shortages Background . 2 H2RA Alternatives . 2 Therapeutic Alternatives . 2 Adults . 2 GERD . 3 PUD . 3 Pediatrics . 3 GERD . 3 PUD . 4 Tables Table 1: Health Canada–Approved Indications of H2RAs . 2 Table 2: Oral Adult Doses of H2RAs and PPIs for GERD . 4 Table 3: Oral Adult Doses of H2RAs and PPIs for PUD . 5 Table 4: Oral Pediatric Doses of H2RAs and PPIs for GERD . 6 Table 5: Oral Pediatric Doses of H2RAs and PPIs for PUD . 7 References . 8 H2-receptor antagonist (H2RA) Shortages | May 25, 2020 1 H2-receptor antagonist (H2RA) Shortages BACKGROUND Health Canada recalls1 and manufacturer supply disruptions may be causing shortages of commonly used acid-reducing medications called histamine H2-receptor antagonists (H2RAs) . H2RAs include cimetidine, famotidine, nizatidine and ranitidine . 2 There are several Health Canada–approved indications of H2RAs (see Table 1); this document addresses the most common: gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD) . 2 TABLE 1: HEALTH CANADA–APPROVED INDICATIONS OF H2RAs H -Receptor Antagonists (H RAs) Health Canada–Approved Indications 2 2 Cimetidine Famotidine Nizatidine Ranitidine Duodenal ulcer, treatment ü ü ü ü Duodenal ulcer, prophylaxis — ü ü ü Benign gastric ulcer, treatment ü ü ü ü Gastric ulcer, prophylaxis — — — ü GERD, treatment ü ü ü ü GERD, maintenance of remission — ü — — Gastric hypersecretion,* treatment ü ü — ü Self-medication of acid indigestion, treatment and prophylaxis — ü† — ü† Acid aspiration syndrome, prophylaxis — — — ü Hemorrhage from stress ulceration or recurrent bleeding, — — — ü prophylaxis ü = Health Canada–approved indication; GERD = gastroesophageal reflux disease *For example, Zollinger-Ellison syndrome . -

Depot Antipsychotic Injections
Mental Health Services Good Practice Statement For The Use Of DEPOT & LONG ACTING ANTIPSYCHOTIC INJECTIONS Date of Revision: February 2020 Approved by: Mental Health Prescribing Management Group Review Date: CONSULTATION The document was sent to Community Mental Health teams, Pharmacy and the Psychiatric Advisory Committee for comments prior to completion. KEY DOCUMENTS When reading this policy please consult the NHS Consent Policy when considering any aspect of consent National Institute for Clinical Excellence CG178 (2014), Psychosis and schizophrenia in adults: prevention and management NHS Lothian (2011), Depot Antipsychotic Guidance, Maudsley (2015), Prescribing Guidelines in Psychiatry 12th edition. UKPPG (2009) Guidance on the Administration to Adults of Oil based Depot and other Long Acting Intramuscular Antipsychotic Injections Summary of Product Characteristics for: Clopixol (Zuclopethixol Decanoate) Flupenthixol Decanoate Haldol (Haloperidol Decanoate) Risperdal Consta Xeplion (Paliperidone Palmitate) ZypAdhera (Olanzapine Embonate) Abilify Maintena (Aripiprazole) 1 CONTENTS REFERENCES/KEY DOCUMENTS …………………………………………………………… 1 INTRODUCTION …………………………………………………………………………………. 3 SCOPE …………………………………………………………………………………………….. 3 DEFINITIONS ……………………………………………………………………………………... 3 CHOICE OF DRUG AND DOSAGE SELECTION ……………………………………………. 3 SIDE EFFECTS ……………………………………………………………….………………….. 5 PRESCRIBING …………………………………………………………………………………… 5 ADMINISTRATION ………………………………………………………………………………. 7 RECORDING ADMINISTRATION ……………………………………………………………… 7 -

Deanxit SPC.Pdf
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Deanxit film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Flupentixol 0.5 mg (as 0.584 mg flupentixol dihydrochloride) Melitracen 10 mg (as 11.25 mg melitracen hydrochloride) Excipients with known effect: Lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablets. Round, biconvex, cyclamen, film-coated tablets. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Anxiety Depression Asthenia. Neurasthenia. Psychogenic depression. Depressive neuroses. Masked depression. Psychosomatic affections accompanied by anxiety and apathy. Menopausal depressions. Dysphoria and depression in alcoholics and drug-addicts. 4.2 Posology and method of administration Posology Adults Usually 2 tablets daily: morning and noon. In severe cases the morning dose may be increased to 2 tablets. The maximum dose is 4 tablets daily. Older people (> 65 years) 1 tablet in the morning. In severe cases 1 tablet in the morning and 1 at noon. Maintenance dose: Usually 1 tablet in the morning. SPC Portrait-REG_00051968 20v038 Page 1 of 10 In cases of insomnia or severe restlessness additional treatment with a sedative in the acute phase is recommended. Paediatric population Children and adolescents (<18 years) Deanxit is not recommended for use in children and adolescents due to lack of data on safety and efficacy. Reduced renal function Deanxit can be given in the recommended doses. Reduced liver function Deanxit can be given in the recommended doses. Method of administration The tablets are swallowed with water. 4.3 Contraindications Hypersensitivity to flupentixol and melitracen or to any of the excipients listed in section 6.1. -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief (if Notes / Similar NCF Active? available) Miscellaneous Medications Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized solution Beta2 Agonist Yes Albuterol, metered dose inhaler Beta2 Agonist NPTC Meeting Update *Any product* Yes (MDI) (Nov 2017) Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Inhibitor Gout (2016) Yes Alogliptin Antidiabetic Agent, Dipeptidyl Peptidase 4 (DPP-4) Inhibitor DPP-IV Inhibitors (2019) Yes Anastrozole Antineoplastic Agent, Aromatase Inhibitor Yes Aspirin Antiplatelet Agent; Nonsteroidal Anti-Inflammatory Drug; Salicylate Yes Azithromycin Antibiotic, Macrolide STIs - PART 1 (2021) Yes Calcium Electrolyte supplement *Any formulation* Yes Carbidopa-Levodopa (immediate Anti-Parkinson Agent; Decarboxylase Inhibitor-Dopamine Precursor Parkinson's Disease Yes release) (2019) Clindamycin, topical ===REMOVED from NCF=== (See Benzoyl Peroxide AND Removed January No Clindamycin, topical combination) 2020 Corticosteroid, intranasal Intranasal Corticosteroid *Any product* Yes Cyanocobalamin (Vitamin B12), Vitamin, Water Soluble Hematologic Supplements Yes oral (2016) Printed on 09/25/2021 Page 1 of 18 National Core Formulary; Last Updated: 09/23/2021 Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief