ZANTAC® 150 (Ranitidine Hydrochloride) Tablets, USP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2D6 Substrates 2D6 Inhibitors 2D6 Inducers
Physician Guidelines: Drugs Metabolized by Cytochrome P450’s 1 2D6 Substrates Acetaminophen Captopril Dextroamphetamine Fluphenazine Methoxyphenamine Paroxetine Tacrine Ajmaline Carteolol Dextromethorphan Fluvoxamine Metoclopramide Perhexiline Tamoxifen Alprenolol Carvedilol Diazinon Galantamine Metoprolol Perphenazine Tamsulosin Amiflamine Cevimeline Dihydrocodeine Guanoxan Mexiletine Phenacetin Thioridazine Amitriptyline Chloropromazine Diltiazem Haloperidol Mianserin Phenformin Timolol Amphetamine Chlorpheniramine Diprafenone Hydrocodone Minaprine Procainamide Tolterodine Amprenavir Chlorpyrifos Dolasetron Ibogaine Mirtazapine Promethazine Tradodone Aprindine Cinnarizine Donepezil Iloperidone Nefazodone Propafenone Tramadol Aripiprazole Citalopram Doxepin Imipramine Nifedipine Propranolol Trimipramine Atomoxetine Clomipramine Encainide Indoramin Nisoldipine Quanoxan Tropisetron Benztropine Clozapine Ethylmorphine Lidocaine Norcodeine Quetiapine Venlafaxine Bisoprolol Codeine Ezlopitant Loratidine Nortriptyline Ranitidine Verapamil Brofaramine Debrisoquine Flecainide Maprotline olanzapine Remoxipride Zotepine Bufuralol Delavirdine Flunarizine Mequitazine Ondansetron Risperidone Zuclopenthixol Bunitrolol Desipramine Fluoxetine Methadone Oxycodone Sertraline Butylamphetamine Dexfenfluramine Fluperlapine Methamphetamine Parathion Sparteine 2D6 Inhibitors Ajmaline Chlorpromazine Diphenhydramine Indinavir Mibefradil Pimozide Terfenadine Amiodarone Cimetidine Doxorubicin Lasoprazole Moclobemide Quinidine Thioridazine Amitriptyline Cisapride -

Receptor Antagonist (H RA) Shortages | May 25, 2020 2 2 2 GERD4,5 • Take This Opportunity to Determine If Continued Treatment Is Necessary
H2-receptor antagonist (H2RA) Shortages Background . 2 H2RA Alternatives . 2 Therapeutic Alternatives . 2 Adults . 2 GERD . 3 PUD . 3 Pediatrics . 3 GERD . 3 PUD . 4 Tables Table 1: Health Canada–Approved Indications of H2RAs . 2 Table 2: Oral Adult Doses of H2RAs and PPIs for GERD . 4 Table 3: Oral Adult Doses of H2RAs and PPIs for PUD . 5 Table 4: Oral Pediatric Doses of H2RAs and PPIs for GERD . 6 Table 5: Oral Pediatric Doses of H2RAs and PPIs for PUD . 7 References . 8 H2-receptor antagonist (H2RA) Shortages | May 25, 2020 1 H2-receptor antagonist (H2RA) Shortages BACKGROUND Health Canada recalls1 and manufacturer supply disruptions may be causing shortages of commonly used acid-reducing medications called histamine H2-receptor antagonists (H2RAs) . H2RAs include cimetidine, famotidine, nizatidine and ranitidine . 2 There are several Health Canada–approved indications of H2RAs (see Table 1); this document addresses the most common: gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD) . 2 TABLE 1: HEALTH CANADA–APPROVED INDICATIONS OF H2RAs H -Receptor Antagonists (H RAs) Health Canada–Approved Indications 2 2 Cimetidine Famotidine Nizatidine Ranitidine Duodenal ulcer, treatment ü ü ü ü Duodenal ulcer, prophylaxis — ü ü ü Benign gastric ulcer, treatment ü ü ü ü Gastric ulcer, prophylaxis — — — ü GERD, treatment ü ü ü ü GERD, maintenance of remission — ü — — Gastric hypersecretion,* treatment ü ü — ü Self-medication of acid indigestion, treatment and prophylaxis — ü† — ü† Acid aspiration syndrome, prophylaxis — — — ü Hemorrhage from stress ulceration or recurrent bleeding, — — — ü prophylaxis ü = Health Canada–approved indication; GERD = gastroesophageal reflux disease *For example, Zollinger-Ellison syndrome . -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

IHS National Pharmacy & Therapeutics Committee National
IHS National Pharmacy & Therapeutics Committee National Core Formulary; Last Updated: 09/23/2021 **Note: Medications in GREY indicate removed items.** Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief (if Notes / Similar NCF Active? available) Miscellaneous Medications Acetaminophen Analgesic, Miscellaneous Yes Albuterol nebulized solution Beta2 Agonist Yes Albuterol, metered dose inhaler Beta2 Agonist NPTC Meeting Update *Any product* Yes (MDI) (Nov 2017) Alendronate Bisphosphonate Derivative Osteoporosis (2016) Yes Allopurinol Antigout Agent; Xanthine Oxidase Inhibitor Gout (2016) Yes Alogliptin Antidiabetic Agent, Dipeptidyl Peptidase 4 (DPP-4) Inhibitor DPP-IV Inhibitors (2019) Yes Anastrozole Antineoplastic Agent, Aromatase Inhibitor Yes Aspirin Antiplatelet Agent; Nonsteroidal Anti-Inflammatory Drug; Salicylate Yes Azithromycin Antibiotic, Macrolide STIs - PART 1 (2021) Yes Calcium Electrolyte supplement *Any formulation* Yes Carbidopa-Levodopa (immediate Anti-Parkinson Agent; Decarboxylase Inhibitor-Dopamine Precursor Parkinson's Disease Yes release) (2019) Clindamycin, topical ===REMOVED from NCF=== (See Benzoyl Peroxide AND Removed January No Clindamycin, topical combination) 2020 Corticosteroid, intranasal Intranasal Corticosteroid *Any product* Yes Cyanocobalamin (Vitamin B12), Vitamin, Water Soluble Hematologic Supplements Yes oral (2016) Printed on 09/25/2021 Page 1 of 18 National Core Formulary; Last Updated: 09/23/2021 Generic Medication Name Pharmacological Category (up-to-date) Formulary Brief -

Wednesday, July 10, 2019 4:00Pm
Wednesday, July 10, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – July 10, 2019 DATE: July 3, 2019 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the July meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Jornay PM™ [Methylphenidate Extended-Release (ER) Capsule], Evekeo ODT™ [Amphetamine Orally Disintegrating Tablet (ODT)], Adhansia XR™ (Methylphenidate ER Capsule), and Sunosi™ (Solriamfetol Tablet) – Appendix C Action Item – Vote to Prior Authorize Balversa™ (Erdafitinib) – Appendix D Action Item – Vote to Prior Authorize Annovera™ (Segesterone Acetate/Ethinyl Estradiol Vaginal System), Bijuva™ (Estradiol/Progesterone Capsule), Cequa™ (Cyclosporine 0.09% Ophthalmic Solution), Corlanor® (Ivabradine Oral Solution), Crotan™ (Crotamiton 10% Lotion), Gloperba® (Colchicine Oral Solution), Glycate® (Glycopyrrolate Tablet), Khapzory™ (Levoleucovorin Injection), Qmiiz™ ODT [Meloxicam Orally Disintegrating Tablet (ODT)], Seconal Sodium™ (Secobarbital -

CLINICAL REVIEW(S) Clinical Review Michael C
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 210655Orig1s000 CLINICAL REVIEW(S) Clinical Review Michael C. Davis, MD, PhD and Qi Chen, MD, MPH (Safety Review) NDA 210655 Perseris (RBP-7000 risperidone-ATRIGEL) CLINICAL REVIEW Application Type Initial 505(b)(2) New Drug Application (NDA) Application Number(s) 210655 Priority or Standard Standard Submit Date(s) September 28, 2017 Received Date(s) September 28, 2017 PDUFA Goal Date July 28, 2018 Division/Office Division of Psychiatry Products/Office of Drug Evaluation I Reviewer Name(s) Michael C. Davis, MD, PhD; Qi Chen, MD, MPH (Safety Review) Review Completion Date June 22, 2018 Established/Proper Name RBP-7000 (risperidone-ATRIGEL) (Proposed) Trade Name Perseris Applicant Indivior Dosage Form(s) Injectable Suspension Applicant Proposed Dosing 90 mg or 120 mg subcutaneous injection monthly Regimen(s) Applicant Proposed Schizophrenia/Adults Indication(s)/Population(s) Recommendation on Approve Regulatory Action Recommended N/A Indication(s)/Population(s) (if applicable) CDER Clinical Review Template 1 Version date: September 6, 2017 for all NDAs and BLAs Reference ID: 4288016 Clinical Review Michael C. Davis, MD, PhD and Qi Chen, MD, MPH (Safety Review) NDA 210655 Perseris (RBP-7000 risperidone-ATRIGEL) Table of Contents Glossary ........................................................................................................................................... 9 1. Executive Summary .............................................................................................................. -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -
Effervescent H2 Blocker Formulation
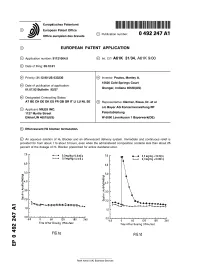
Europaisches Patentamt European Patent Office © Publication number: 0 492 247 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91121064.9 © int. CIA A61K 31/34, A61K 9/00 @ Date of filing: 09.12.91 ® Priority: 21.12.90 US 633230 @ Inventor: Paulos, Manley A. 15626 Cold Springs Court @ Date of publication of application: Indiana 01.07.92 Bulletin 92/27 Granger, 46530(US) © Designated Contracting States: AT BE CH DE DK ES FR GB GR IT LI LU NL SE © Representative: Danner, Klaus, Dr. et al c/o Bayer AG Konzernverwaltung RP © Applicant: MILES INC. 1127 Myrtle Street Patentabteilung ElkhartJN 4651 5(US) W-5090 Leverkusen 1 Bayerwerk(DE) (§v Effervescent H2 blocker formulation. © An aqueous solution of H2 Blocker and an effervescent delivery system. Immediate and continuous relief is provided for from about 1 to about 3 hours, even when the administered composition contains less than about 25 percent of the dosage of H2 Blocker prescribed for active duodenal ulcer. 7.0 r 0,3mg/kg+0,0mEq 7.0 0,3 mg/kg + 32mEq 0,0mg/kg+0,0mEq 0,0mg/kg +0,0mE q 6.0 6,0 5,0 5,0 Xa. *4.0 :4.0 ^3.0 :3,0 1^2,0 h is 2,0 1.0 h 1.0 ■ 0.0 _i i ■ ■ 0.0 CM -60 0 60 120 180 240 -60 0 60 120 180 240 Time After Dosing (Minutes) CM Time After Dosing (Minutes) Oi FIG.1Q FIG.Id Rank Xerox (UK) Business Services EP 0 492 247 A1 Background of the Invention Histamine H2 receptor blocking agents (referred to herein as H2 Blockers) are a class of drugs which act as antagonists of the histamine H2 receptor. -

Appendix 1 BNF Codes of Drugs Lists Used to Define Exposure Groups
Appendix 1 BNF Codes of drugs lists used to define exposure groups. Anticholinergic antipsychotics d411. Chlorpromazine d412. Chlorpromazine d413. Chlorpromazine d414. Chlorpromazine d415. Chlorpromazine d41a. Chlorpromazine d41b. Chlorpromazine d41c. Chlorpromazine d41d. Chlorpromazine d4b1. Perphenazine d4e1. PROMAZINE d4ex. PROMAZINE d4g.. Thioridazine d4g1. Thioridazine d4g2. Thioridazine d4g3. Thioridazine d4g5. Thioridazine d4g7. Thioridazine d4gp. Thioridazine d4gt. Thioridazine d4gu. Thioridazine d4gv. Thioridazine d4gw. Thioridazine d4gz. Thioridazine d4h.. Trifluoperazine d4h1. Trifluoperazine d4h2. Trifluoperazine d4h3. Trifluoperazine d4h4. Trifluoperazine d4hs. Trifluoperazine d4ht. Trifluoperazine d4hu. Trifluoperazine d4hx. Trifluoperazine d4l2. CLOZAPINE d4r1. OLANZAPINE d4r3. OLANZAPINE d4r7. OLANZAPINE d4s1. QUETIAPINE d4s2. QUETIAPINE d4s3. QUETIAPINE d4s5. QUETIAPINE d4ss. QUETIAPINE d4sx. QUETIAPINE Tricyclic antidepressants d7... d71.. Amitriptyline d711. Amitriptyline d712. Amitriptyline d713. Amitriptyline d719. Amitriptyline d71a. Amitriptyline d71b. Amitriptyline d71c. Amitriptyline d71d. Amitriptyline d71e. Amitriptyline d71f. Amitriptyline d71u. Amitriptyline d71v. Amitriptyline d71w. Amitriptyline d71y. Amitriptyline d71z. Amitriptyline d73.. Clomipramine d731. Clomipramine d732. Clomipramine d733. Clomipramine d736. Clomipramine d73s. Clomipramine d73t. Clomipramine d73u. Clomipramine d73v. Clomipramine d73w. Clomipramine d73z. Clomipramine d75.. DOSULEPIN d751. DOSULEPIN d752. DOSULEPIN d755. DOSULEPIN d756. -

Carvedilol Suppresses Intractable Hiccups
J Am Board Fam Med: first published as 10.3122/jabfm.19.4.418 on 29 June 2006. Downloaded from BRIEF REPORTS Carvedilol Suppresses Intractable Hiccups Danielle Stueber, MD, and Conrad M. Swartz, PhD, MD Carvedilol (6.25 mg, 4 times daily) relieved 2 years of constant hiccupping, marked tardive dyskinesia, compulsive self-induced vomiting, and feelings of hopelessness and low mood in a 59-year-old African- American man. He previously failed trials of ranitidine, chlorpromazine, promethazine, tegaserod, on- dansetron, metoclopramide, pantoprazole, pyloric injections of botulinum toxin A, and a vagal nerve stimulator. At a 5-month follow-up, improvement was maintained; there had been several instances of rapid relapse on carvedilol discontinuation. (J Am Board Fam Med 2006;19:418–21.) This report describes a case of persistent and in- Case Reports tractable postoperative hiccups of 2 years duration The patient was a 59-year-old African-American that responded to carvedilol after nonresponse to man, admitted to the hospital for nausea, frequent typical therapies. The chronic singultus was one of coffee ground hematemesis, and associated anemia, several concurrent pathologic conditions, including besides unrelenting hiccups. Hiccups began episod- self-induced vomiting, tardive dyskinesia secondary ically 10 years prior. These episodes were initially to metoclopramide use, and depressed mood. relieved by self-induced vomiting and attributed to Although major causes of hiccups are associated diabetic gastroparesis. The patient underwent sev- with gastrointestinal ailments, persistent hiccups eral upper endoscopic examinations, which re- can be induced by tumors, chemotherapy, diabetes, vealed gastric erosions, a 3-cm hiatal hernia, and a uremia, or brain disease. -

Early Effects of Oral Administration of Omeprazole and Roxatidine on Intragastric Ph
Iida et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012 13(1):29-34 29 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus; www.springerlink.com E-mail: [email protected] Early effects of oral administration of omeprazole and roxatidine on intragastric pH Hiroshi IIDA1, Shingo KATO1, Yusuke SEKINO1, Eiji SAKAI1, Takashi UCHIYAMA1, Hiroki ENDO1, Kunihiro HOSONO1, Yasunari SAKAMOTO1, Koji FUJITA1, Masato YONEDA1, Tomoko KOIDE1, Hirokazu TAKAHASHI1, Chikako TOKORO1, Ayumu GOTO1, Yasunobu ABE1, Noritoshi KOBAYASHI1, Kensuke KUBOTA1, Eiji GOTOH2, Shin MAEDA1, Atsushi NAKAJIMA1, Masahiko INAMORI†‡1,3 (1Gastroenterology Division, Yokohama City University School of Medicine, Yokohama 236-0004, Japan) (2Department of Medical Education, Yokohama City University School of Medicine, Yokohama 236-0004, Japan) (3Office of Postgraduate Medical Education, Yokohama City University Hospital, Yokohama 236-0004, Japan) †E-mail: [email protected] Received Mar. 14, 2011; Revision accepted Aug. 30, 2011; Crosschecked Dec. 2, 2011 Abstract: Objective: The ideal medication for the treatment of acid-related diseases, e.g., peptic ulcers, stress- related gastric bleeding, functional dyspepsia, and gastroesophageal reflux disease, should have a rapid onset of action to promote hemostasis and relieve the symptoms. The aim of our study was to investigate the inhibitory effects on gastric acid secretion of a single oral administration of a proton pump inhibitor, omeprazole 20 mg, and an H2-receptor antagonist, roxatidine 75 mg. Methods: Ten Helicobacter pylori-negative male subjects participated in this randomized, two-way crossover study. Intragastric pH was monitored continuously for 6 h after single oral admini- stration of omeprazole 20 mg and roxatidine 75 mg. -

Should Inverse Agonists Be Defined by Pharmacological Mechanism Or
CNS Spectrums,(2019), 24 354 – 370. © Cambridge University Press 2018. This is an Open Access article, distributed under the terms , of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is unaltered and is properly cited. The written permission of Cambridge University Press must be obtained for commercial re-use or in order to create a derivative work. doi:10.1017/S1092852918001098 REVIEW ARTICLE Practical considerations for managing breakthrough psychosis and symptomatic worsening in patients with schizophrenia on long-acting injectable antipsychotics Christoph U. Correll ,1,2 Jennifer Kern Sliwa ,3* Dean M. Najarian ,3 and Stephen R. Saklad4 1 Department of Psychiatry and Molecular Medicine, Hofstra Northwell School of Medicine, Hempstead, New York 2 Division of Psychiatry Research, Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York 3 Janssen Scientific Affairs LLC, Titusville, New Jersey 4 College of Pharmacy, Pharmacotherapy Division, University of Texas at Austin, San Antonio, Texas With more long-acting injectable (LAI) antipsychotics available for treating schizophrenia, each with variable durations of action (2 weeks to 3 months), it is important to have clear management strategies for patients developing breakthrough psychotic symptoms or experiencing symptomatic worsening on LAIs. However, no treatment guidelines or clinical practice pathways exist; health-care providers must rely on their own clinical judgment to manage these patients. This article provides practical recommendations—based on a framework of clinical, pharmacokinetic, and dosing considerations—to guide clinicians’ decisions regarding management of breakthrough psychotic symptoms.