Improved Forensic Toxicology Screening Using a GC/MS/NPD System with a 725-Compound DRS Database
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Considerations in Perioperative Assessment of Valproic Acid Coagulopathy Claude Abdallah George Washington University
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Anesthesiology and Critical Care Medicine Faculty Anesthesiology and Critical Care Medicine Publications 1-2014 Considerations in perioperative assessment of valproic acid coagulopathy Claude Abdallah George Washington University Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_anesth_facpubs Part of the Anesthesia and Analgesia Commons APA Citation Abdallah, C. (2014). Considerations in perioperative assessment of valproic acid coagulopathy. Journal of Anaesthesiology Clinical Pharmacology, Volume 30, Issue 1 (). http://dx.doi.org/10.4103/0970-9185.125685 This Journal Article is brought to you for free and open access by the Anesthesiology and Critical Care Medicine at Health Sciences Research Commons. It has been accepted for inclusion in Anesthesiology and Critical Care Medicine Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. [Downloaded free from http://www.joacp.org on Tuesday, February 25, 2014, IP: 128.164.86.61] || Click here to download free Android application for this journal Revv iew Article Considerations in perioperative assessment of valproic acid coagulopathy Claude Abdallah Department of Anesthesiology, Children’s National Medical Center, The George Washington University Medical Center, NW Washington, DC, USA Abstract Valproic acid (VPA) is one of the widely prescribed antiepileptic drugs in children with multiple indications. VPA-induced coagulopathy may occur and constitute a pharmacological and practical challenge affecting pre-operative evaluation and management of patients receiving VPA therapy. This review summarizes the different studies documenting the incidence, severity and available recommendations related to this adverse effect. -

Journal of Pharmacology and Experimental Therapeutics
Journal of Pharmacology and Experimental Therapeutics Molecular Determinants of Ligand Selectivity for the Human Multidrug And Toxin Extrusion Proteins, MATE1 and MATE-2K Bethzaida Astorga, Sean Ekins, Mark Morales and Stephen H Wright Department of Physiology, University of Arizona, Tucson, AZ 85724, USA (B.A., M.M., and S.H.W.) Collaborations in Chemistry, 5616 Hilltop Needmore Road, Fuquay-Varina NC 27526, USA (S.E.) Supplemental Table 1. Compounds selected by the common features pharmacophore after searching a database of 2690 FDA approved compounds (www.collaborativedrug.com). FitValue Common Name Indication 3.93897 PYRIMETHAMINE Antimalarial 3.3167 naloxone Antidote Naloxone Hydrochloride 3.27622 DEXMEDETOMIDINE Anxiolytic 3.2407 Chlordantoin Antifungal 3.1776 NALORPHINE Antidote Nalorphine Hydrochloride 3.15108 Perfosfamide Antineoplastic 3.11759 Cinchonidine Sulfate Antimalarial Cinchonidine 3.10352 Cinchonine Sulfate Antimalarial Cinchonine 3.07469 METHOHEXITAL Anesthetic 3.06799 PROGUANIL Antimalarial PROGUANIL HYDROCHLORIDE 100MG 3.05018 TOPIRAMATE Anticonvulsant 3.04366 MIDODRINE Antihypotensive Midodrine Hydrochloride 2.98558 Chlorbetamide Antiamebic 2.98463 TRIMETHOPRIM Antibiotic Antibacterial 2.98457 ZILEUTON Antiinflammatory 2.94205 AMINOMETRADINE Diuretic 2.89284 SCOPOLAMINE Antispasmodic ScopolamineHydrobromide 2.88791 ARTICAINE Anesthetic 2.84534 RITODRINE Tocolytic 2.82357 MITOBRONITOL Antineoplastic Mitolactol 2.81033 LORAZEPAM Anxiolytic 2.74943 ETHOHEXADIOL Insecticide 2.64902 METHOXAMINE Antihypotensive Methoxamine -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Aminothiazolones As Estrogen Related
(19) TZZ ¥__T (11) EP 2 536 716 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 417/06 (2006.01) C07D 417/14 (2006.01) 21.05.2014 Bulletin 2014/21 A61K 31/427 (2006.01) A61P 5/30 (2006.01) (21) Application number: 11705404.9 (86) International application number: PCT/US2011/024999 (22) Date of filing: 16.02.2011 (87) International publication number: WO 2011/103130 (25.08.2011 Gazette 2011/34) (54) AMINOTHIAZOLONES AS ESTROGEN RELATED RECEPTOR-ALPHA MODULATORS AMINOTHIAZOLONE ALS ERR-ALPHA-MODULATOREN AMINOTHIAZOLONES EN TANT QUE MODULATEURS DE RÉCEPTEUR ALPHA ASSOCIÉ AUX STROGÈNES (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • BIGNAN, Gilles GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Spring House, PA 19477 (US) PL PT RO RS SE SI SK SM TR • GAUL, Micheal Designated Extension States: Spring House, PA 19477 (US) BA ME • XU, Guozhang Spring House, PA 19477 (US) (30) Priority: 17.02.2010 US 305177 P •ZHAO,Bao- ping Spring House, PA 19477 (US) (43) Date of publication of application: 26.12.2012 Bulletin 2012/52 (74) Representative: Carpmaels & Ransford LLP One Southampton Row (73) Proprietor: Janssen Pharmaceutica, N.V. London WC1B 5HA (GB) 2340 Beerse (BE) (56) References cited: WO-A1-2008/109737 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Lega Italiana Per La Lotta Contro La Malattia Di Parkinson Le Sindromi Extrapiramidali E Le Demenze
LIMPE lega italiana per la lotta contro la malattia di parkinson le sindromi extrapiramidali e le demenze Web site: http://www.parkinson-limpe.it/ XXIX National Congress Lecce 23–25 October 2002 Parkinson Parkinsonism Dementia: from Biotechnology to Medical Management SELECTED SHORT PAPERS Scientific Committee Bruno Bergamasco, Giovanni U. Corsini, Mario Manfredi, Stefano Ruggieri, Pierfranco Spano Secretariat LIMPE Viale dell’Università 30, 00185 Roma Tel. and Fax: +39-06-4455618 E-mail: [email protected] Neurol Sci (2003) 24:149–150 DOI 10.1007/s10072-003-0103-5 123I-Ioflupane/SPECT binding to to the dopamine transporter (DAT) may be better suited and provide more-accurate estimation of degeneration. Several striatal dopamine transporter (DAT) tracers that bind to DAT and utilize SPECT are available; uptake in patients with Parkinson’s they are all cocaine derivatives. The most widely used are disease, multiple system atrophy, and [123I]β-CIT and 123I-Ioflupane ([123I]FP-CIT) [3, 4]. The main advantage of 123I-Ioflupane is that a steady state allow- progressive supranuclear palsy ing SPECT imaging is reached 3 h after a single bolus injec- 1 2 1 tion of the radioligand, compared with the 18–24 h required A. Antonini (౧) • R. Benti • R. De Notaris 1 1 1 for [123I]β-CIT. Therefore DAT imaging with 123I-Ioflupane S. Tesei • A. Zecchinelli • G. Sacilotto 1 1 1 1 can be completed the same day. N. Meucci • M. Canesi • C. Mariani • G. Pezzoli 123 P. Gerundini2 Several studies have demonstrated the usefulness of I- 1 Centro per la malattia di Parkinson, Dipartimento di Ioflupane SPECT imaging in the diagnosis of parkinsonism. -
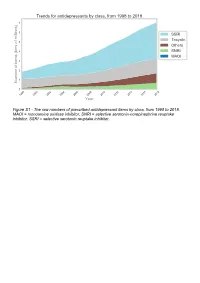
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 6-2020 The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting Robert J. Kohler Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Biological Psychology Commons Recommended Citation Kohler, Robert J., "The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting" (2020). Dissertations. 3565. https://scholarworks.wmich.edu/dissertations/3565 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING by Robert J. Kohler A dissertation submitted to the Graduate College In partial fulfillment of the requirements for the degree of Doctor of Philosophy Psychology Western Michigan University June 2020 Doctoral Committee: Lisa Baker, Ph.D., Chair Anthony DeFulio, Ph.D. Cynthia Pietras, Ph.D. John Spitsbergen, Ph.D. Copyright by Robert J. Kohler 2020 THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING Robert J. Kohler, Ph.D. Western Michigan University, 2020 The resurgence of Lysergic Acid Diethylamide (LSD) as a therapeutic tool requires a revival in research, both basic and clinical, to bridge gaps in knowledge left from a previous generation of work. Currently, no study has been published with the intent of establishing optimal microdose concentrations of LSD in an animal model.