Glomerular Epithelial Stem Cells: the Good, the Bad, and the Ugly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-Stage Invasive Colorectal Carcinoma
ANTICANCER RESEARCH 25: 2705-2712 (2005) Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-stage Invasive Colorectal Carcinoma PIN LIANG1, JIAN-WEI HONG2, HIDEYUKI UBUKATA1, GE LIU1, MOTONOBU KATANO1, GYO MOTOHASHI1, TERUHIKO KASUGA1, YOSHINORI WATANABE1, ICHIRO NAKADA1 and TAKAFUMI TABUCHI1 1Fourth Department of Surgery and 2Department of Pathology, Tokyo Medical University Kasumigaura Hospital, Ibaraki, Japan Abstract. Background: Recent studies have shown that the Myofibroblasts are the main component cells in tumor interactions between tumor cells and stromal cells are stroma, and alpha-smooth muscle actin (·-SMA)-positive important in tumor development. A possible correlation myofibroblasts were found to participate in the synthesis of between tumor-activated myofibroblasts, the main component extracellular matrix components of tumor stroma, and to cells of tumor stroma, and lymphatic microvessel density produce lytic enzymes able to degrade the basement (LMVD) or other clinical parameters in carcinoma was membrane surrounding tumor glands. Although present in investigated. Materials and Methods: Immunohistochemical the progressive tumor nodules, they disappear during tumor examination of alpha-smooth muscle actin and podoplanin regression (5, 6). The correlation between microvessel were performed in 83 cases of early-stage invasive colorectal density and myofibroblasts was shown by Zidar et al. (7), but carcinoma. Results: There was a good correlation between the influence of myofibroblasts in lymphagiogenesis remains proliferation of myofibroblasts (PMpt) and LMVD (LMVDpt) unclear. Only recently, podoplanin, a 43-kd glomerular in the peri-tumoral area (p=0.0034). Increased PMpt was also podocyte membrane mucoprotein and a specific lymphatic associated with lymphatic invasion (p=0.0051) and with vessel marker, has enabled the investigation of the lymph node metastasis (p=0.011). -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Podocytes Are Firmly Attached to Glomerular Basement Membrane in Kidneys with Heavy Proteinuria
J Am Soc Nephrol 15: 2611–2618, 2004 Podocytes Are Firmly Attached to Glomerular Basement Membrane in Kidneys with Heavy Proteinuria ANNE-TIINA LAHDENKARI,* KARI LOUNATMAA,† JAAKKO PATRAKKA,*‡ CHRISTER HOLMBERG,* JORMA WARTIOVAARA,§ MARJO KESTILA¨ ,ʈ OLLI KOSKIMIES,* and HANNU JALANKO* *Hospital for Children and Adolescents and Biomedicum Helsinki, University of Helsinki, Helsinki, Finland; †Helsinki University of Technology, Laboratory of Electronics Production Technology, Espoo, Finland; ‡Division of Matrix Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institut, Stockholm, Sweden; §Electron Microscopy Unit, Institute of Biotechnology, University of Helsinki, Helsinki, Finland; and ʈDepartment of Molecular Medicine, National Public Health Institute, Helsinki, Finland Abstract. Glomerular epithelial cells (podocytes) play an im- in the basal and apical parts of the podocytes was comparable portant role in the pathogenesis of proteinuria. Podocyte foot in proteinuric and control kidneys; (4) in proteinuric kidneys, process effacement is characteristic for proteinuric kidneys, the podocyte slit pore density was decreased by 69 to 80% and and genetic defects in podocyte slit diaphragm proteins may up to half of the slits were so “tight” that no visible space cause nephrotic syndrome. In this work, a systematic electron between foot processes was seen; thus, the filtration surface microscopic analysis was performed of the structural changes area between podocytes was dramatically reduced; and (5)in of podocytes in two important nephrotic kidney diseases, con- the narrow MCNS slit pores, nephrin was located in the apical genital nephrotic syndrome of the Finnish type and minimal- part of the podocyte foot process, indicating vertical transfer of change nephrotic syndrome (MCNS). The results showed that the slit diaphragm complex in proteinuria. -

(A) Adrenal Gland Inferior Vena Cava Iliac Crest Ureter Urinary Bladder
Hepatic veins (cut) Inferior vena cava Adrenal gland Renal artery Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female Urinary reproductive bladder system) Urethra (a) © 2018 Pearson Education, Inc. 1 12th rib (b) © 2018 Pearson Education, Inc. 2 Renal cortex Renal column Major calyx Minor calyx Renal pyramid (a) © 2018 Pearson Education, Inc. 3 Cortical radiate vein Cortical radiate artery Renal cortex Arcuate vein Arcuate artery Renal column Interlobar vein Interlobar artery Segmental arteries Renal vein Renal artery Minor calyx Renal pelvis Major calyx Renal Ureter pyramid Fibrous capsule (b) © 2018 Pearson Education, Inc. 4 Cortical nephron Fibrous capsule Renal cortex Collecting duct Renal medulla Renal Proximal Renal pelvis cortex convoluted tubule Glomerulus Juxtamedullary Ureter Distal convoluted tubule nephron Nephron loop Renal medulla (a) © 2018 Pearson Education, Inc. 5 Proximal convoluted Peritubular tubule (PCT) Glomerular capillaries capillaries Distal convoluted tubule Glomerular (DCT) (Bowman’s) capsule Efferent arteriole Afferent arteriole Cells of the juxtaglomerular apparatus Cortical radiate artery Arcuate artery Arcuate vein Cortical radiate vein Collecting duct Nephron loop (b) © 2018 Pearson Education, Inc. 6 Glomerular PCT capsular space Glomerular capillary covered by podocytes Efferent arteriole Afferent arteriole (c) © 2018 Pearson Education, Inc. 7 Filtration slits Podocyte cell body Foot processes (d) © 2018 Pearson Education, Inc. 8 Afferent arteriole Glomerular capillaries Efferent Cortical arteriole radiate artery Glomerular 1 capsule Three major renal processes: Rest of renal tubule 11 Glomerular filtration: Water and solutes containing smaller than proteins are forced through the filtrate capillary walls and pores of the glomerular capsule into the renal tubule. Peritubular 2 capillary 2 Tubular reabsorption: Water, glucose, amino acids, and needed ions are 3 transported out of the filtrate into the tubule cells and then enter the capillary blood. -

Urine-Derived Epithelial Cells As Models for Genetic Kidney Diseases
cells Review Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases Tjessa Bondue 1 , Fanny O. Arcolino 1 , Koenraad R. P. Veys 1,2, Oyindamola C. Adebayo 1,3, Elena Levtchenko 1,2, Lambertus P. van den Heuvel 1,4 and Mohamed A. Elmonem 5,* 1 Department of Development and Regeneration, KU Leuven, 3000 Leuven, Belgium; [email protected] (T.B.); [email protected] (F.O.A.); [email protected] (K.R.P.V.); [email protected] (O.C.A.); [email protected] (E.L.); [email protected] (L.P.v.d.H.) 2 Department of Pediatrics, Division of Pediatric Nephrology, University Hospitals Leuven, 3000 Leuven, Belgium 3 Centre for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, 3000 Leuven, Belgium 4 Department of Pediatric Nephrology, Radboud University Medical Center, 6500 Nijmegen, The Netherlands 5 Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Cairo 11628, Egypt * Correspondence: [email protected] Abstract: Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much Citation: Bondue, T.; Arcolino, F.O.; interest. In this review, we summarize the methodology for establishing human podocyte and PTEC Veys, K.R.P.; Adebayo, O.C.; cell lines from urine and highlight their importance as kidney disease cell models. -
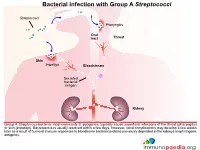
Bacterial Infection with Group a Streptococci
Bacterial infection with Group A Streptococci Streptococci Pharyngitis Oral tract Throat Skin Impetigo Bloodstream Secreted bacterial antigen Kidney Group A Streptoccus bacteria, most commonly S. pyogenes, typically cause superficial infections of the throat (pharyngitis) or skin (impetigo). Bacteraemia is usually resolved within a few days, however, renal complications may develop a few weeks later as a result of humoral immune responses to bloodborne bacterial proteins previously deposited in the kidneys (nephritogenic antigens). Innate and adaptive immune response to Streptococcal infection Secreted bacterial antigen Streptococci Skin Antibody Phagocytosis help CD4+ helper T cell Macrophage Neutrophil B cell Kidney An important factor in the development of kidney dysfunction following a Streptococcal infection is the generation of antibodies that target the secreted bacterial antigens. These antibodies are produced during initial immune responses to bacterial infection when immune cells such as macrophages, neutrophils, CD4+ helper T cells and B cells are recruited to the site of infection. Immune complex formation and deposition in the kidneys Secreted bacterial antigen Antibody Immune complex formation Kidney When antibodies have been produced in abundance by plasma cells, antibody-antigen complexes can form in the bloodstream and become deposited in the kidneys, or unbound antobodies can detect antigen in the kidney and from “in situ” immune complexes. Structure of kidney nephron Nephron Afferent Distal arteriole convoluted Efferent tubule arteriole Cortex Kidney Renal corpuscle Proximal convoluted tubule Medulla Loop of Henle The normal filtering of waste products from the blood is performed by thousands of nephron structures located in the kidney where renal corpuscles are responsible for allowing small molecules to diffuse out of the capillaries. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

N-Type Calcium Channel Inhibition with Cilnidipine Elicits Glomerular Podocyte Protection Independent of Sympathetic Nerve Inhibition
J Pharmacol Sci 119, 359 – 367 (2012) Journal of Pharmacological Sciences © The Japanese Pharmacological Society Full Paper N-type Calcium Channel Inhibition With Cilnidipine Elicits Glomerular Podocyte Protection Independent of Sympathetic Nerve Inhibition Bai Lei1, Daisuke Nakano1,*, Yoshihide Fujisawa1, Ya Liu1, Hirofumi Hitomi1, Hiroyuki Kobori1, Hirohito Mori2, Tsutomu Masaki2, Katsuhiko Asanuma3, Yasuhiko Tomino3, and Akira Nishiyama1 1Department of Pharmacology, 2Department of Gastroenterology, Kagawa University Medical School, Kagawa 761-0793, Japan 3Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, Tokyo 113-8421, Japan Received March 25, 2012; Accepted June 17, 2012 Abstract. We recently demonstrated that cilnidipine, an L/N-type calcium channel blocker, elicits protective effects against glomerular podocyte injury, in particular, in obese hypertensive rats that express the N-type calcium channel (N-CC). Since the N-CC is known to be expressed in sympathetic nerve endings, we evaluated the reno-protective effects of cilnidipine in innervated and denervated spontaneously hypertensive rats (SHR). Male SHR were uninephrectomized and fed 4% high-salt diet (HS-UNX-SHR). Animals were divided into groups, as follows, and observed from 9 to 27 weeks of age: 1) vehicle (n = 14), 2) vehicle plus renal-denervation (n = 15), 3) cilnidipine (50 mg/kg per day, p.o.; n = 10), and 4) cilnidipine plus renal-denervation (n = 15). Renal denervation attenuated elevations in blood pressure, but failed to suppress urinary protein excretion and podocyte injury in HS-UNX-SHR. Cilnidipine in both innervated and denervated HS-UNX-SHR similarly induced significant antihypertensive effects, as well as suppressing the urinary protein excretion and podocyte injury, compared to vehicle-treated HS-UNX-SHR. -

Molecular Mechanisms of Podocyte Development Revealed By
lopmen ve ta e l B D io & l l o l g e y C Cell & Developmental Biology Wingert, et al., Cell Dev Biol 2014, 3:2 ISSN: 2168-9296 DOI: 10.4172/2168-9296.1000138 Review Article Open Access Molecular Mechanisms of Podocyte Development Revealed by Zebrafish Kidney Research Miceli R1, Kroeger PT1 and Wingert RA1,2* 1Department of Biological Sciences and Center for Zebrafish Research, University of Notre Dame, Notre Dame, IN 46556, USA 2Department of Biological Sciences, University of Notre, Dame, 100 Galvin Life Sciences, Notre Dame, USA *Corresponding author: Rebecca A Wingert, Department of Biological Sciences, University of Notre, Dame, 100 Galvin Life Sciences, Notre Dame, USA, Phone: (574)-631-907; Fax: (574)-631-741; E-mail: [email protected] Rec date: Apr 13, 2014, Acc date: Jun 05, 2014, Pub date: Jun 07, 2014 Copyright: © 2014 Wingert RA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Elucidating the gene regulatory networks that control kidney development can provide information about the origins of renal birth defects and kidney disease, as well as insights relevant to the design of clinical interventions for these conditions. The kidney is composed of functional units termed nephrons. Renal malfunction often arises from damage to cells known as podocytes, which are highly specialized epithelial cells that comprise the blood filter, or glomerulus, located on each nephron. Podocytes interact with the vasculature to create an elaborate sieve that collects circulatory fluid, and this filtrate enters the nephron where it is modified to produce urine and balance water homeostasis.