Manipulation of Nephron-Patterning Signals Enables Selective Induction of Podocytes from Human Pluripotent Stem Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-Stage Invasive Colorectal Carcinoma
ANTICANCER RESEARCH 25: 2705-2712 (2005) Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-stage Invasive Colorectal Carcinoma PIN LIANG1, JIAN-WEI HONG2, HIDEYUKI UBUKATA1, GE LIU1, MOTONOBU KATANO1, GYO MOTOHASHI1, TERUHIKO KASUGA1, YOSHINORI WATANABE1, ICHIRO NAKADA1 and TAKAFUMI TABUCHI1 1Fourth Department of Surgery and 2Department of Pathology, Tokyo Medical University Kasumigaura Hospital, Ibaraki, Japan Abstract. Background: Recent studies have shown that the Myofibroblasts are the main component cells in tumor interactions between tumor cells and stromal cells are stroma, and alpha-smooth muscle actin (·-SMA)-positive important in tumor development. A possible correlation myofibroblasts were found to participate in the synthesis of between tumor-activated myofibroblasts, the main component extracellular matrix components of tumor stroma, and to cells of tumor stroma, and lymphatic microvessel density produce lytic enzymes able to degrade the basement (LMVD) or other clinical parameters in carcinoma was membrane surrounding tumor glands. Although present in investigated. Materials and Methods: Immunohistochemical the progressive tumor nodules, they disappear during tumor examination of alpha-smooth muscle actin and podoplanin regression (5, 6). The correlation between microvessel were performed in 83 cases of early-stage invasive colorectal density and myofibroblasts was shown by Zidar et al. (7), but carcinoma. Results: There was a good correlation between the influence of myofibroblasts in lymphagiogenesis remains proliferation of myofibroblasts (PMpt) and LMVD (LMVDpt) unclear. Only recently, podoplanin, a 43-kd glomerular in the peri-tumoral area (p=0.0034). Increased PMpt was also podocyte membrane mucoprotein and a specific lymphatic associated with lymphatic invasion (p=0.0051) and with vessel marker, has enabled the investigation of the lymph node metastasis (p=0.011). -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
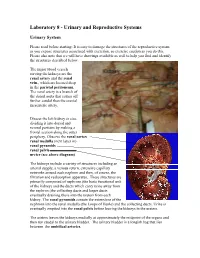
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Podocytes Are Firmly Attached to Glomerular Basement Membrane in Kidneys with Heavy Proteinuria
J Am Soc Nephrol 15: 2611–2618, 2004 Podocytes Are Firmly Attached to Glomerular Basement Membrane in Kidneys with Heavy Proteinuria ANNE-TIINA LAHDENKARI,* KARI LOUNATMAA,† JAAKKO PATRAKKA,*‡ CHRISTER HOLMBERG,* JORMA WARTIOVAARA,§ MARJO KESTILA¨ ,ʈ OLLI KOSKIMIES,* and HANNU JALANKO* *Hospital for Children and Adolescents and Biomedicum Helsinki, University of Helsinki, Helsinki, Finland; †Helsinki University of Technology, Laboratory of Electronics Production Technology, Espoo, Finland; ‡Division of Matrix Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institut, Stockholm, Sweden; §Electron Microscopy Unit, Institute of Biotechnology, University of Helsinki, Helsinki, Finland; and ʈDepartment of Molecular Medicine, National Public Health Institute, Helsinki, Finland Abstract. Glomerular epithelial cells (podocytes) play an im- in the basal and apical parts of the podocytes was comparable portant role in the pathogenesis of proteinuria. Podocyte foot in proteinuric and control kidneys; (4) in proteinuric kidneys, process effacement is characteristic for proteinuric kidneys, the podocyte slit pore density was decreased by 69 to 80% and and genetic defects in podocyte slit diaphragm proteins may up to half of the slits were so “tight” that no visible space cause nephrotic syndrome. In this work, a systematic electron between foot processes was seen; thus, the filtration surface microscopic analysis was performed of the structural changes area between podocytes was dramatically reduced; and (5)in of podocytes in two important nephrotic kidney diseases, con- the narrow MCNS slit pores, nephrin was located in the apical genital nephrotic syndrome of the Finnish type and minimal- part of the podocyte foot process, indicating vertical transfer of change nephrotic syndrome (MCNS). The results showed that the slit diaphragm complex in proteinuria. -

(A) Adrenal Gland Inferior Vena Cava Iliac Crest Ureter Urinary Bladder
Hepatic veins (cut) Inferior vena cava Adrenal gland Renal artery Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female Urinary reproductive bladder system) Urethra (a) © 2018 Pearson Education, Inc. 1 12th rib (b) © 2018 Pearson Education, Inc. 2 Renal cortex Renal column Major calyx Minor calyx Renal pyramid (a) © 2018 Pearson Education, Inc. 3 Cortical radiate vein Cortical radiate artery Renal cortex Arcuate vein Arcuate artery Renal column Interlobar vein Interlobar artery Segmental arteries Renal vein Renal artery Minor calyx Renal pelvis Major calyx Renal Ureter pyramid Fibrous capsule (b) © 2018 Pearson Education, Inc. 4 Cortical nephron Fibrous capsule Renal cortex Collecting duct Renal medulla Renal Proximal Renal pelvis cortex convoluted tubule Glomerulus Juxtamedullary Ureter Distal convoluted tubule nephron Nephron loop Renal medulla (a) © 2018 Pearson Education, Inc. 5 Proximal convoluted Peritubular tubule (PCT) Glomerular capillaries capillaries Distal convoluted tubule Glomerular (DCT) (Bowman’s) capsule Efferent arteriole Afferent arteriole Cells of the juxtaglomerular apparatus Cortical radiate artery Arcuate artery Arcuate vein Cortical radiate vein Collecting duct Nephron loop (b) © 2018 Pearson Education, Inc. 6 Glomerular PCT capsular space Glomerular capillary covered by podocytes Efferent arteriole Afferent arteriole (c) © 2018 Pearson Education, Inc. 7 Filtration slits Podocyte cell body Foot processes (d) © 2018 Pearson Education, Inc. 8 Afferent arteriole Glomerular capillaries Efferent Cortical arteriole radiate artery Glomerular 1 capsule Three major renal processes: Rest of renal tubule 11 Glomerular filtration: Water and solutes containing smaller than proteins are forced through the filtrate capillary walls and pores of the glomerular capsule into the renal tubule. Peritubular 2 capillary 2 Tubular reabsorption: Water, glucose, amino acids, and needed ions are 3 transported out of the filtrate into the tubule cells and then enter the capillary blood.