Claudins in the Renal Collecting Duct
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

(TRPV6) EXPRESSION in RABBIT GUT EPITHELIUM RANJAN R.*, DAS P.*, BATABYAL S.†, MINJ A.P.* *Department of Veterinary Anatomy, Faculty of Veterinary and Animal Sciences
W orld World Rabbit Sci. 2020, 28: 187-197 R abbit doi:10.4995/wrs.2020.12161 Science WRSA, UPV, 2003 PATTERNS OF CALCIUM CHANNEL (TRPV6) EXPRESSION IN RABBIT GUT EPITHELIUM RANJAN R.*, DAS P.*, BATABYAL S.†, MINJ A.P.* *Department of Veterinary Anatomy, Faculty of Veterinary and Animal Sciences. West Bengal University of Animal and Fishery Sciences, Kolkata-700 037, West Bengal, India. †Department of Veterinary Biochemistry, Faculty of Veterinary and Animal Sciences. West Bengal University of Animal and Fishery Sciences, Kolkata-700 037, West Bengal, India. Abstract: The present study was undertaken to explore the immunohistochemical localisation of TRPV6 calcium channels in rabbit gut epithelium that are actively involved in calcium absorption. To undertake the research, twelve apparently healthy adult female rabbits with a body weight between 1.0 to 1.5 kg were procured, acclimatised and divided into two groups: control and test. Both groups were kept on same feed along with exogenous calcium supplementation in test group animals only. The serum calcium level revealed that normally a high value of serum calcium is maintained in the rabbit as compared to other mammals, thus indicating that the homeostatic mechanism might be poorly developed. Immunohistochemistry and reverse transcription polymerase chain reaction analysis revealed that the caecum was the site of maximum calcium absorption in rabbit, followed by the duodenum and jejunum. The expression pattern of TRPV6 protein/mRNA was weaker in test group animals than in the control group, indicating that the channel was functional in low calcium concentration in the gut. Key Words: rabbit, gut epithelium, TRPV6, immunohistochemistry, RT-PCR. -

ZOOLOGY Animal Physiology Osmoregulation in Aquatic
Paper : 06 Animal Physiology Module : 27 Osmoregulation in Aquatic Vertebrates Development Team Principal Investigator: Prof. Neeta Sehgal Department of Zoology, University of Delhi Co-Principal Investigator: Prof. D.K. Singh Department of Zoology, University of Delhi Paper Coordinator: Prof. Rakesh Kumar Seth Department of Zoology, University of Delhi Content Writer: Dr Haren Ram Chiary and Dr. Kapinder Kirori Mal College, University of Delhi Content Reviewer: Prof. Neeta Sehgal Department of Zoology, University of Delhi 1 Animal Physiology ZOOLOGY Osmoregulation in Aquatic Vertebrates Description of Module Subject Name ZOOLOGY Paper Name Zool 006: Animal Physiology Module Name/Title Osmoregulation Module Id M27:Osmoregulation in Aquatic Vertebrates Keywords Osmoregulation, Active ionic regulation, Osmoconformers, Osmoregulators, stenohaline, Hyperosmotic, hyposmotic, catadromic, anadromic, teleost fish Contents 1. Learning Objective 2. Introduction 3. Cyclostomes a. Lampreys b. Hagfish 4. Elasmobranches 4.1. Marine elasmobranches 4.2. Fresh-water elasmobranches 5. The Coelacanth 6. Teleost fish 6.1. Marine Teleost 6.2. Fresh-water Teleost 7. Catadromic and anadromic fish 8. Amphibians 8.1. Fresh-water amphibians 8.2. Salt-water frog 9. Summary 2 Animal Physiology ZOOLOGY Osmoregulation in Aquatic Vertebrates 1. Learning Outcomes After studying this module, you shall be able to • Learn about the major strategies adopted by different aquatic vertebrates. • Understand the osmoregulation in cyclostomes: Lamprey and Hagfish • Understand the mechanisms adopted by sharks and rays for osmotic regulation • Learn about the strategies to overcome water loss and excess salt concentration in teleosts (marine and freshwater) • Analyse the mechanisms for osmoregulation in catadromic and anadromic fish • Understand the osmotic regulation in amphibians (fresh-water and in crab-eating frog, a salt water frog). -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -
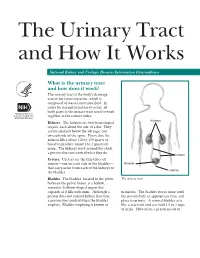
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

The Osmotic Adjustment of Marine Microalgae
The osmotic adjustment of marine microalgae and the complex role osmolytes play in this process Thesis of Liz Kendall Supervisor Dr. Gieskes Department of Marine Biology, The University of Groningen April 1996 D5O Summary: Algae inhabit a wide variety of both marine and freshwater habitats. These habitats differ in regard to various factors such as chemical composition, the organisms that live there, the light which may radiate into that particular area, the temperature of the sites depending on where the environment is located, just to name a few. One factor that varies from environment to environment is the salinity. This paper will look at the mechanisms utilized by marine algae to cope with the changes in salinity content in their habitats and most importantly how they use different osmolytes to carry out this process. Marine algae "osmotically adjust" themselves to external salinity changes, in a biphasic maimer. Firstly, this includes changes in turgor pressure or large internal water fluxes in response to osmotic gradients. Secondly. an internally regulated osmotic adjustment occurs with the use of both inorganic and organic osmolytes. Compatible solutes are ions and molecules used by man organisms to osmotically adjust and they play a double role in the process of osmotic adjustment. They act as osmolytes and also protect the cellular enzymatic activities under salinity stress. They are called 'compatible solutes" because they protect the cellular enzymatic activity. The main compatible solutes are polyols (including amino acids, carbohydrates and sugars). quaternary ammonium derivatives or tertiary sulphonium compounds. Certain species and taxonomic classes use specific compatible solutes and some even use combinations of them. -

Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M
14 The Open Dermatology Journal, 2010, 4, 14-20 Open Access Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M. Brandner1,§, Marek Haftek*,2,§ and Carien M. Niessen3,§ 1Department of Dermatology and Venerology, University Hospital Hamburg-Eppendorf, Hamburg, Germany 2University of Lyon, EA4169 Normal and Pathological Functions of Skin Barrier, E. Herriot Hospital, Lyon, France 3Department of Dermatology, Center for Molecular Medicine, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Germany Abstract: The skin is an indispensable barrier which protects the body from the uncontrolled loss of water and solutes as well as from chemical and physical assaults and the invasion of pathogens. In recent years several studies have suggested an important role of intercellular junctions for the barrier function of the epidermis. In this review we summarize our knowledge of the impact of adherens junctions, (corneo)-desmosomes and tight junctions on barrier function of the skin. Keywords: Cadherins, catenins, claudins, cell polarity, stratum corneum, skin diseases. INTRODUCTION ADHERENS JUNCTIONS The stratifying epidermis of the skin physically separates Adherens junctions are intercellular structures that couple the organism from its environment and serves as its first line intercellular adhesion to the cytoskeleton thereby creating a of structural and functional defense against dehydration, transcellular network that coordinate the behavior of a chemical substances, physical insults and micro-organisms. population of cells. Adherens junctions are dynamic entities The living cell layers of the epidermis are crucial in the and also function as signal platforms that regulate formation and maintenance of the barrier on two different cytoskeletal dynamics and cell polarity. -

New Approaches to Studies of Paracellular Drug Transport in Intestinal Epithelial Cell Monolayers
&RPSUHKHQVLYH6XPPDULHVRI8SSVDOD'LVVHUWDWLRQV IURPWKH)DFXOW\RI3KDUPDF\ 1HZ$SSURDFKHVWR6WXGLHVRI 3DUDFHOOXODU'UXJ7UDQVSRUWLQ,QWHVWLQDO (SLWKHOLDO&HOO0RQROD\HUV %< 67$))$17$9(/,1 $&7$81,9(56,7$7,6836$/,(16,6 8336$/$ Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutics presented at Uppsala University in 2003 ABSTRACT Tavelin, S., 2003. New Approaches to Studies of Paracellular Drug Transport in Intestinal Epithelial Cell Monolayers. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 285. 66 pp. Uppsala. ISBN 91-554-5582-4. Studies of intestinal drug permeability have traditionally been performed in the colon-derived Caco-2 cell model. However, the paracellular permeability of these cell monolayers resembles that of the colon rather than that of the small intestine, which is the major site of drug absorption following oral administration. One aim of this thesis was therefore to develop a new cell culture model that mimics the permeability of the small intestine. 2/4/A1 cells are conditionally immortalized with a temperature sensitive mutant of SV40T. These cells proliferate and form multilayers at 33°C. At cultivation temperatures of 37–39°C, they stop proliferating and form monolayers. 2/4/A1 cells cultivated on permeable supports expressed functional tight junctions. The barrier properties of the tight junctions such as transepithelial electrical resistance and permeability to hydrophilic paracellular markers resembled those of the human small intestine in vivo. These cells lacked functional expression of drug transport proteins and can therefore be used as a model to study passive drug permeability unbiased by active transport. The permeability to diverse sets of drugs in 2/4/A1 was comparable to that of the human jejunum for both incompletely and completely absorbed drugs, and the prediction of human intestinal permeability was better in 2/4/A1 than in Caco-2 for incompletely absorbed drugs. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
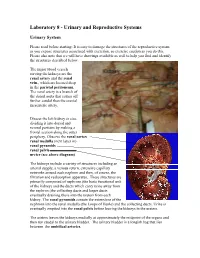
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Morphology of the Kidney in a Nectarivorous Bird, the Anna's Hummingbird Calypte Anna
J. Zool., Lond. (1998) 244, 175±184 # 1998 The Zoological Society of London Printed in the United Kingdom Morphology of the kidney in a nectarivorous bird, the Anna's hummingbird Calypte anna G. Casotti1*, C. A. Beuchat2 and E. J. Braun3 1 Department of Biology, West Chester University, West Chester, PA, 19383, U.S.A. 2 Department of Biology, San Diego State University, San Diego, CA, 92182, U.S.A. 3 Department of Physiology, Arizona Health Sciences Center, University of Arizona, P.O. Box 245051, Tucson, AZ, 85724±5051, U.S.A. (Accepted 21 April 1997) Abstract The kidneys of Anna's hummingbird (Calypte anna) differ in several signi®cant ways from those of other birds that have been examined. The kidneys of this nectarivore contain very little medullary tissue; 90% of the total volume of the kidneys is cortical tissue, with medulla accounting for only an additional 2%. More than 99% of the nephrons are the so-called `reptilian type', which lack the loop of Henle. The few looped (`mammalian type') nephrons are incorporated into only a few medullary cones per kidney. The loopless nephrons are similar to those of other birds. However, the looped nephrons differ in that they lack the thin descending limb of the loop of Henle, which is found in other birds and is thought to play an important role in the countercurrent multiplier system in the avian kidney. Instead, the cells of the nephron segment following the pars recta of the proximal tubule resemble those of the thick ascending limb, with the large populations of mitochondria that are typical of transporting epithelia and no reduction in cell height.