Urine-Derived Epithelial Cells As Models for Genetic Kidney Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Aquaporin Channels in the Heart—Physiology and Pathophysiology
International Journal of Molecular Sciences Review Aquaporin Channels in the Heart—Physiology and Pathophysiology Arie O. Verkerk 1,2,* , Elisabeth M. Lodder 2 and Ronald Wilders 1 1 Department of Medical Biology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 2 Department of Experimental Cardiology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +31-20-5664670 Received: 29 March 2019; Accepted: 23 April 2019; Published: 25 April 2019 Abstract: Mammalian aquaporins (AQPs) are transmembrane channels expressed in a large variety of cells and tissues throughout the body. They are known as water channels, but they also facilitate the transport of small solutes, gasses, and monovalent cations. To date, 13 different AQPs, encoded by the genes AQP0–AQP12, have been identified in mammals, which regulate various important biological functions in kidney, brain, lung, digestive system, eye, and skin. Consequently, dysfunction of AQPs is involved in a wide variety of disorders. AQPs are also present in the heart, even with a specific distribution pattern in cardiomyocytes, but whether their presence is essential for proper (electro)physiological cardiac function has not intensively been studied. This review summarizes recent findings and highlights the involvement of AQPs in normal and pathological cardiac function. We conclude that AQPs are at least implicated in proper cardiac water homeostasis and energy balance as well as heart failure and arsenic cardiotoxicity. However, this review also demonstrates that many effects of cardiac AQPs, especially on excitation-contraction coupling processes, are virtually unexplored. -

Control of Fluid Absorption in the Renal Proximal Tubule
Control of fluid absorption in the renal proximal tubule Maurice B. Burg, Jack Orloff J Clin Invest. 1968;47(9):2016-2024. https://doi.org/10.1172/JCI105888. Research Article Glomerulotubular balance was investigated in isolated, perfused rabbit proximal tubules in vitro in order to evaluate some of the mechanisms proposed to account for the proportionate relationship between glomerular filtration rate and fluid absorption generally observed in vivo. The rate of fluid transport from lumen to bath in proximal convoluted tubules in vitro was approximately equal to the estimated normal rate in vivo. The absorption rate in proximal straight tubules however was approximately one-half as great. If the mechanism responsible for maintenance of glomerulotubular balance is intrinsic to the proximal tubule, as has been proposed on the basis of micropuncture studies, the rate of fluid absorption in vitro should be directly related to the perfusion rate and/or tubule volume. In the present studies absorption rate was only minimally affected when perfusion rate was increased or the tubule distended. Thus, glomerulotubular balance is not mediated by changes in velocity of flow of the tubular fluid or tubular diameter and therefore is not an intrinsic property of the proximal tubule. It has also been proposed that glomerulotubular balance results from a humoral feedback mechanism in which angiotensin directly inhibits fluid absorption by the proximal convoluted tubule. In the present experiments, angiotensin was found to have no significant effect on absorption rate. Find the latest version: https://jci.me/105888/pdf Control of Fluid Absorption in the Renal Proximal Tubule MAURICE B. -
![L5 6 -Renal Reabsorbation and Secretation [PDF]](https://docslib.b-cdn.net/cover/2118/l5-6-renal-reabsorbation-and-secretation-pdf-252118.webp)
L5 6 -Renal Reabsorbation and Secretation [PDF]
Define tubular reabsorption, Identify and describe tubular secretion, Describe tubular secretion mechanism involved in transcellular and paracellular with PAH transport and K+ Glucose reabsorption transport. Identify and describe Identify and describe the Study glucose titration curve mechanisms of tubular characteristic of loop of in terms of renal threshold, transport & Henle, distal convoluted tubular transport maximum, Describe tubular reabsorption tubule and collecting ducts splay, excretion and filtration of sodium and water for reabsorption and secretion Identify the tubular site and Identify the site and describe Revise tubule-glomerular describe how Amino Acids, the influence of aldosterone feedback and describe its HCO -, P0 - and Urea are on reabsorption of Na+ in the physiological importance 3 4 reabsorbed late distal tubules. Mind Map As the glomerular filtrate enters the renal tubules, it flows sequentially through the successive parts of the tubule: The proximal tubule → the loop of Henle(1) → the distal tubule(2) → the collecting tubule → finally ,the collecting duct, before it is excreted as urine. A long this course, some substances are selectively reabsorbed from the tubules back into the blood, whereas others are secreted from the blood into the tubular lumen. The urine represent the sum of three basic renal processes: glomerular filtration, tubular reabsorption, and tubular secretion: Urinary excretion = Glomerular Filtration – Tubular reabsorption + Tubular secretion Mechanisms of cellular transport in the nephron are: Active transport Pinocytosis\ Passive Transport Osmosis “Active transport can move a solute exocytosis against an electrochemical gradient and requires energy derived from metabolism” Water is always reabsorbed by a Simple diffusion passive (nonactive) (Additional reading) Primary active (without carrier physical mechanism Secondary active The proximal tubule, reabsorb protein) called osmosis , transport large molecules such as transport Cl, HCO3-, urea , which means water proteins by pinocytosis. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

As of May 16, 2021 Member List
As Of May 16, 2021 Member List First Middle Last Designation Website Clarice Nell Aaron Genevieve Abate http://genabate333.fineartstudioonline.com Lynn Abbott http://www.lynnabbottstudios.com Kim Abernethy http://www.kimabernethy.com Liz Abeyta http://www.lizabeyta.com Bryan Abing Susan Abma http://www.susanabma.com Sharon Abshagen http://www.sharonabshagenart.com Angela Marie Accarino http://www.sunfishart.com Benone Achiriloaie Sandra Ackerman Ed Acuna Amy Adams http://www.Amyadamsfineart.com Michael Lynn Adams http://www.MichaelLynnAdams.com Peter Adams OPAM http://www.Americanlegacyfinearts.com Warren W. Adams http://www.wyomingartist.net Dustin Adamson http://www.dustinadamsonfineart.com Robert N. Adamson OPA Teresa Adaszynska http://www.adaszynskafineart.com Cyrus Afsary OPAM Priya Ahlawat http://www.priyaahlawat.com Susan Aitcheson Michael Aitken Robert T. Akers http://www.robertakers.com Robert Lawrence Akey http://www.robakey.com Daud Akhriev OPAM http://www.daudakhriev.com Lee Alban OPA http://www.leealban.com Ann H. Aldinger https://www.annaldinger.com Christy Carroll Aldredge http://www.christyaldredge.com Edward Aldrich OPA http://www.edwardaldrich.com Anne Aleshire http://www.annealeshire.com Ann Alexander Charles David Alexander http://www.charlesdavidalexander.com Gloria Alexander Joseph Alexander http://www.josephalexanderstudio.com Richard Alexander http://www.richalexanderart.com Stanton deForest Allaben http://www.stantonallabenart.com Martha Allan Laverty http://www.marthalaverty.com Laura Lawson Allee http://www.llawsonallee.faso.com/ -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -
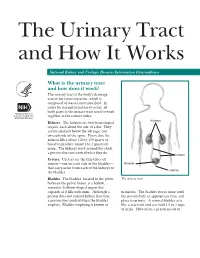
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -
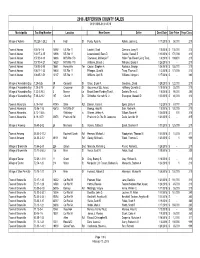
2018 Sales Report
2018 JEFFERSON COUNTY SALES 01/01/2018-01/31/2018 Municipality Tax Map NumberLocation New Owner Seller Deed Date Sale Price Prop Class Village of Adams 112.28-1-28.2 15 High St Purdy, Ryan A. Rohde, Joanne L 1/17/2018$ 58,000 210 Town of Adams 100.14-1-6 14592 US Rte 11 Laisdell, Scott Clemons, Larry R 1/8/2018$ 135,000 210 Town of Adams 100.17-2-15 13906 US Rte 11 Lewandowski, Sean D. Gaulke, Russell S 1/10/2018$ 170,000 210 Town of Adams 107.00-4-13 16686 NYS Rte 178 Townsend, McKenzie F. Haller Fam Revoc Living Trust, 1/8/2018$ 169,600 210 Town of Adams 107.00-4-21 16520 NYS Rte 178 Williams, Brandi J. Williams, Brandi J 1/26/2018$ - 210 Town of Adams 108.05-2-80 18661 Honeyville Ter Casion, Stephen A. Roukous, George 1/26/2018$ 155,000 210 Town of Adams 108.17-1-33 10604 US Rte 11 Pfleegor, Chad M. Tharp, Thomas S 1/3/2018$ 173,000 210 Town of Adams 108.05-1-51 13137 US Rte 11 Williams, April R. Williams, Morgan J 1/17/2018$ - 280 Village of Alexandria Bay 7.29-3-26 39 Cornwall St Olson, Bryan R. Goudreau, David 1/29/2018$ 122,000 210 Village of Alexandria Bay 7.29-3-78 81 Crossmon St Gouverneur S&L Assoc, Hoffberg, Camette C 1/18/2018$ 25,000 210 Village of Alexandria Bay 7.22-2-29.3 3 Mance Ln Broad Street Funding Trust I, Daniels, Emma L 1/5/2018$ 95,000 280 Village of Alexandria Bay 7.38-2-21.2 107 Church St Schreiber, Kenneth M. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Allosteric Mechanism of Water Channel Gating by Ca2+–Calmodulin
Portland State University PDXScholar Chemistry Faculty Publications and Presentations Chemistry 9-2013 Allosteric Mechanism of Water Channel Gating by Ca2+–calmodulin Steve Reichow [email protected] Daniel M. Clemens University of California, Irvine J. Alfredo Freites University of California, Irvine Karin L. Németh-Cahalan University of California, Irvine Matthias Heyden University of California, Irvine See next page for additional authors Let us know how access to this document benefits ouy . Follow this and additional works at: https://pdxscholar.library.pdx.edu/chem_fac Part of the Biochemistry Commons, and the Structural Biology Commons Citation Details Reichow, S. L., Clemens, D. M., Freites, J. A., Németh-Cahalan, K. L., Heyden, M., Tobias, D. J., ... & Gonen, T. (2013). Allosteric mechanism of water-channel gating by Ca2+–calmodulin. Nature structural & molecular biology, 20(9), 1085-1092. This Post-Print is brought to you for free and open access. It has been accepted for inclusion in Chemistry Faculty Publications and Presentations by an authorized administrator of PDXScholar. For more information, please contact [email protected]. Authors Steve Reichow, Daniel M. Clemens, J. Alfredo Freites, Karin L. Németh-Cahalan, Matthias Heyden, Douglas J. Tobias, James E. Hall, and Tamir Gonen This post-print is available at PDXScholar: https://pdxscholar.library.pdx.edu/chem_fac/198 HHS Public Access Author manuscript Author Manuscript Author ManuscriptNat Struct Author Manuscript Mol Biol. Author Author Manuscript manuscript; available in PMC 2014 March 01. Published in final edited form as: Nat Struct Mol Biol. 2013 September ; 20(9): 1085–1092. doi:10.1038/nsmb.2630. Allosteric mechanism of water channel gating by Ca2+– calmodulin Steve L.