Different Localization and Regulation of Two Types of Vasopressin Receptor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

DDAVP Nasal Spray Is Provided As an Aqueous Solution for Intranasal Use
DDAVP® Nasal Spray (desmopressin acetate) Rx only DESCRIPTION DDAVP® Nasal Spray (desmopressin acetate) is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows: Mol. wt. 1183.34 Empirical formula: C46H64N14O12S2•C2H4O2•3H2O 1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate. DDAVP Nasal Spray is provided as an aqueous solution for intranasal use. Each mL contains: Desmopressin acetate 0.1 mg Sodium Chloride 7.5 mg Citric acid monohydrate 1.7 mg Disodium phosphate dihydrate 3.0 mg Benzalkonium chloride solution (50%) 0.2 mg The DDAVP Nasal Spray compression pump delivers 0.1 mL (10 mcg) of DDAVP (desmopressin acetate) per spray. CLINICAL PHARMACOLOGY DDAVP contains as active substance desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin. One mL (0.1 mg) of intranasal DDAVP has an antidiuretic activity of about 400 IU; 10 mcg of desmopressin acetate is equivalent to 40 IU. 1. The biphasic half-lives for intranasal DDAVP were 7.8 and 75.5 minutes for the fast and slow phases, compared with 2.5 and 14.5 minutes for lysine vasopressin, another form of the hormone used in this condition. As a result, intranasal DDAVP provides a prompt onset of antidiuretic action with a long duration after each administration. 1 2. The change in structure of arginine vasopressin to DDAVP has resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses are usually below threshold levels for effects on vascular or visceral smooth muscle. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Corticotropin-Releasing Activity of Lysine Vasopressin Evelyn Joyce Weber Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1961 Corticotropin-releasing activity of lysine vasopressin Evelyn Joyce Weber Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biochemistry Commons Recommended Citation Weber, Evelyn Joyce, "Corticotropin-releasing activity of lysine vasopressin " (1961). Retrospective Theses and Dissertations. 1990. https://lib.dr.iastate.edu/rtd/1990 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 62-1374 microfilmed exactly as received WEBER, Evelyn Joyce, 1928- CORTICOTRO PIN-RE LEASING ACTIVITY OF LYSINE VASOPRESSIN. Iowa State University of Science and Technology Ph.D., 1961 Chemistry, biological University Microfilms, Inc., Ann Arbor, Michigan CORTICO TROPIN-RELEASING ACTIVITY OF LYSINE 7AS0PRESSII Evelyn Joyce Weber A Dissertation Submitted, to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY . kajor Subject: Biochemistry Approved: Signature was redacted for privacy. In Charge of l-.ejor V,rork Signature was redacted for privacy. Head, of kajor Department Signature was redacted for privacy. Deacf of Gradu/ue College Iowa State University Of Science and Technology Ames, loua 1961 il TABLE OF CONTENTS Page HISTORICAL . ; 1 Biological Activities of Vasopressin. ...... -3 Pressor activity 3 Antidiuretic activity 5 Oxytocic activity. £ Corticotropin releasing activity 10 Other activities of vasopressin. -

Management and Treatment of Lithium-Induced Nephrogenic Diabetes Insipidus
REVIEW Management and treatment of lithium- induced nephrogenic diabetes insipidus Christopher K Finch†, Lithium carbonate is a well documented cause of nephrogenic diabetes insipidus, with as Tyson WA Brooks, many as 10 to 15% of patients taking lithium developing this condition. Clinicians have Peggy Yam & Kristi W Kelley been well aware of lithium toxicity for many years; however, the treatment of this drug- induced condition has generally been remedied by discontinuation of the medication or a †Author for correspondence Methodist University reduction in dose. For those patients unresponsive to traditional treatment measures, Hospital, Department several pharmacotherapeutic regimens have been documented as being effective for the of Pharmacy, University of management of lithium-induced diabetes insipidus including hydrochlorothiazide, Tennessee, College of Pharmacy, 1265 Union Ave., amiloride, indomethacin, desmopressin and correction of serum lithium levels. Memphis, TN 38104, USA Tel.: +1 901 516 2954 Fax: +1 901 516 8178 [email protected] Lithium carbonate is well known for its wide use associated with a mutation(s) of vasopressin in bipolar disorders due to its mood stabilizing receptors. Acquired causes are tubulointerstitial properties. It is also employed in aggression dis- disease (e.g., sickle cell disease, amyloidosis, orders, post-traumatic stress disorders, conduct obstructive uropathy), electrolyte disorders (e.g., disorders and even as adjunctive therapy in hypokalemia and hypercalcemia), pregnancy, or depression. Lithium has many well documented conditions induced by a drug (e.g., lithium, adverse effects as well as a relatively narrow ther- demeclocycline, amphotericin B and apeutic range of 0.4 to 0.8 mmol/l. Clinically vincristine) [3,4]. Lithium is the most common significant adverse effects include polyuria, mus- cause of drug-induced nephrogenic DI [5]. -

VASOPRESSIN and OXYTOCIN Molecular, Cellular, and Clinical Advances ADVANCES in EXPERIMENTAL MEDICINE and BIOLOGY
VASOPRESSIN AND OXYTOCIN Molecular, Cellular, and Clinical Advances ADVANCES IN EXPERIMENTAL MEDICINE AND BIOLOGY Editorial Board: NATHAN BACK, State University of New York at Buffalo IRUN R. COHEN, The Weizmann Institute of Science DAVID KRITCHEVSKY, Wistar Institute ABEL LAJTHA, N. S. Kline Institutefor Psychiatric Research RODOLFO PAOLETTI, University of Milan Recent Volumes in this Series Volume 443 ADV ANCES IN LACTOFERRIN RESEARCH Edited by Genevieve Spik, Dominique Legrand, Joel Mazurier, Annick Pierce, and Jean-Paul Perraudin Volume 444 REPRODUCTIVE TOXICOLOGY: In Vitro Germ Cell Developmental Toxicology, from Science to Social and Industrial Demand Edited by Jesus del Mazo Volume 445 MA THEMA TICAL MODELING IN EXPERIMENTAL NUTRITION Edited by Andrew J. Clifford and Hans-Georg MUlier Volume 446 MOLECULAR AND CELLULAR MECHANISMS OF NEURONAL PLASTICITY: Basic and Clinical Implications Edited by Yigal H. Ehrlich Volume 447 LIPOXYGENASES AND THEIR METABOLITES: Biological Functions Edited by Santosh Nigam and Cecil R. Pace-Asciak Volume 448 COPPER TRANSPORT AND ITS DISORDERS: Molecular aIfd Cellular Aspects Edited by Arturo Leone and Julian F. B. Mercer Volume 449 VASOPRESSIN AND OXYTOCIN: Molecular, Cellular, and Clinical Advances Edited by Harts H.Zingg, Charles W. Bourque, and Daniel G. Bichet Volume 450 ADV ANCES IN MODELING AND CONTROL OF VENTILATION Edited by Richard L. Hughson, David A. Cunningham, and James Duffin Volume 451 GENE THERAPY OF CANCER Edited by Peter Walden, Uwe Trefzer, Wolfram Sterry, and Farzin Farzaneh Volume 452 MECHANISMS OF LYMPHOCYTE ACTIVATION AND IMMUNE REGULATION VII: Molecular Determinants of Microbial Immunity Edited by Sudhir Gupta, Alan Sher, and Rafi Ahmed A Continuation Order Plan is available for this series. -

Oral Desmopressin in Central Diabetes Insipidus
Arch Dis Child: first published as 10.1136/adc.61.3.247 on 1 March 1986. Downloaded from Archives of Disease in Childhood, 1986, 61, 247-250 Oral desmopressin in central diabetes insipidus U WESTGREN, C WITTSTROM, AND A S HARRIS Department of Pediatrics, University Hospital, Lund, and Faculty of Pharmacy, Biomedicum, Uppsala, Sweden SUMMARY Seven paediatric patients with central diabetes insipidus were studied in an open dose ranging study in hospital followed by a six month study on an outpatient basis to assess the efficacy and safety of peroral administration of DDAVP (desmopressin) tablets. In the dose ranging study a dose dependent antidiuretic response was observed. The response to 12-5-50 mcg was, however, less effective in correcting baseline polyuria than were doses of 100 mcg and above. Patients were discharged from hospital on a preliminary dosage regimen ranging from 100 to 400 mcg three times daily. After an initial adjustment in dosage in three patients at one week follow up, all patients were stabilised on treatment with tablets and reported an adequate water turnover at six months. As with the intranasal route of administration dosage requirements varied from patient to patient, and a dose range rather than standard doses were required. A significant correlation, however, was found for the relation between previous intranasal and present oral daily dosage. No adver-se reactions were reported. No clinically significant changes were noted in blood chemistry and urinalysis. All patients expressed a preference for the oral over existing intranasal copyright. treatment. Treatment with tablets offers a beneficial alternative to the intranasal route, particularly in patients with chronic rhinitis or impaired vision. -

Demeclocycline in the Treatment of the Syndrome of Inappropriate Secretion of Antidiuretic Hormone
Thorax: first published as 10.1136/thx.34.3.324 on 1 June 1979. Downloaded from Thorax, 1979, 34, 324-327 Demeclocycline in the treatment of the syndrome of inappropriate secretion of antidiuretic hormone W H PERKS, E H WALTERS,' I P TAMS, AND K PROWSE From the Department of Respiratory Physiology, City General Hospital, Stoke-on-Trent, Staffordshire, UK ABSTRACT Fourteen patients with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) have been treated with demethylchlortetracycline (demeclocycline) 1200 mg daily. In 12 patients the underlying lesion was malignant. The serum sodium returned to normal (> 135 mmol/l) in all patients after a mean of 8-6 days (SD+5-3 days). Blood urea rose significantly from the pretreatment level of 4-2±2-3 mmol/l to 10-1±5-1 mmol/l at ten days (p<0 001). The average maximum blood urea was 13-4-6-8 mmol/l. In four patients the urea rose above 20 mmol/l, and in two of these demecyocycline was discontinued because of this rise. The azotaemia could be attributed to a combination of increased urea production and a mild specific drug-induced nephrotoxicity. Discontinuation of demeclocycline in six patients led to a fall in serum sodium, in one case precipitously, and return of the urea towards normal levels. Demeclocycline appears therefore to be an effective maintenance treatment of SIADH, and the azotaemia that occurs is reversible and probably dose dependent. The syndrome of inappropriate secretion of anti- Methods http://thorax.bmj.com/ diuretic hormone (SIADH) has become increas- ingly recognised as a treatable cause of stupor and Fourteen patients with a diagnosis of SIADH confusion in patients with a wide variety of based on the criteria of De Troyer and Demanet diseases (De Troyer and Demanet, 1976). -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -

Hypernatremia: 2 Months Back When He Had Progressive Decrease in Vision (Right > Left)
Correspondence to increased intrathoracic pressure and central radiologist regarding vascular injury when there is venous pressure, decreased venous return from the refractory hypotension so that necessary steps can be brain and increased ICP[4] taken to identify and treat iatrogenic complications that • Patients with aneurysmal SAH have disturbed might compound the pre‑existing morbidity. autoregulation of cerebral blood flow. Hence, intraoperative hypotension of any cause has an REFERENCES adverse effect on the outcome of SAH[5,6] • Patients with poor grade SAH (Fischer III/IV) and 1. Young WL, Pile-Spellman J. Anesthetic considerations for interventional Neuroradiolgy (Review). Anesthesiology angiographic evidence of vasospasm which our 1994;80:427-56. patient had, are at risk for cerebral hypoperfusion 2. Varma MK, Price K, Jayakrishnan V, Manickam B, Kessell G. because of impaired cerebral autoregulation and Anaesthetic considerations for interventional neuroradiology. thus intraoperative hypotension and cerebral Br J Anaesth 2007;99:75-85. [6,7] 3. Kent KC, Moscucci M, Mansour KA, Dimattia S, Gallagher S, hypoperfusion compounds the poor outcome Kuntz R, et al. Retroperitoneal hematoma after cardiac • Blood loss leading to severe anaemia (Hb 3.8 g%) catheterization: Prevalence, risk factors, and optimal during the procedure might have lead to decreased management. J Vasc Surg 1994;20:905-10. oxygen‑carrying capacity of blood and further 4. De Laet I, Citerio G, Malbrain ML. The influence of exacerbate the hypoxic injury to brain. intraabdominal hypertension on the central nervous system: Current insights and clinical recommendations, is it all in the Thus a combination of age, poor grade SAH, diffuse head? Acta Clin Belg Suppl 2007;1:89-97. -
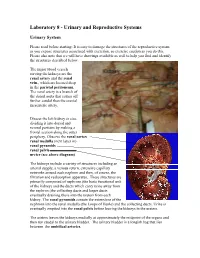
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder.